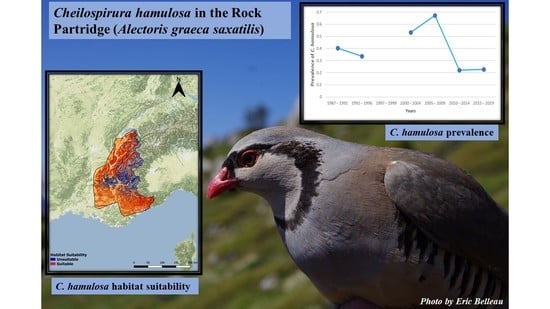
Cheilospirura hamulosa in the Rock Partridge (Alectoris graeca saxatilis): Epidemiological Patterns and Prediction of Parasite Distribution in France
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling and Study Area
2.2. Parasitological Analysis
2.3. Epidemiological Descriptors
2.4. Environmental Variables
2.5. Modelling Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cattadori, I.; Hudson, P.; Merler, S.; Rizzoli, A. Synchrony, scale and temporal dynamics of rock partridge (Alectoris graeca saxatilis) populations in the Dolomites. J. Anim. Ecol. 1999, 68, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Delogu, M.; Ghetti, G.; Gugiatti, A.; Cotti, C.; Piredda, I.; Frasnelli, M.; de Marco, M.A. Virological Investigation of Avian Influenza Virus on Postglacial Species of Phasianidae and Tetraonidae in the Italian Alps. Int. Sch. Res. Not. 2013, 601732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, C. Rock Partridge Alectoris graeca population assessment. In Methodology for Bird Species Recovery Planning in the European Union; Final Report to the European Commission; FACE and BirdLife International for the European Commission: Cambridge, UK, 2011. [Google Scholar]
- BirdLife International. Alectoris graeca. The IUCN Red List of Threatened Species 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22678684A131902869.en (accessed on 4 July 2020).
- UICN France. La Liste Rouge des Espèces Menacées en France; Chapitre Oiseaux de France métropolitaine: Paris, France, 2016. [Google Scholar]
- Bernard-Laurent, A.; de Franceschi, P.F. Status, trends, and limiting factors of Rock Partridge (Alectoris graeca saxatilis) populations. Game Wildl. 1994, 11, 267–307. [Google Scholar]
- Budinski, I.; Čulina, A.; Mikulić, K.; Jurinović, L. Bird species that have significantly changed breeding range on Croatian coastal area: Comparison of 30 years old data and recent knowledge. Bird Census News 2010, 23, 49–58. [Google Scholar]
- Cattadori, I.M.; Ranci-Ortigosa, G.; Gatto, M.; Hudson, P.J. Is the rock partridge Alectoris graeca saxatilis threatened in the Dolomitic Alps? Anim. Conserv. 2003, 6, 71–81. [Google Scholar]
- Manios, N.; Papazahariadou, M.; Frydas, S.; Papageorgiou, N.; Tsachalidis, E.; Gergopoulou, J. Tetrathyridium as a mortality factor of rock partridge (Alectoris graeca graeca) in Central Greece. Eur. J. Wildl. Res. 2002, 48, 378–382. [Google Scholar] [CrossRef]
- Rosà, R.; Bolzoni, L.; Rosso, F..; Pugliese, A.; Hudson, P.J.; Rizzoli, A. Effect of Ascaridia compar infection on rock partridge population dynamics: Empirical and theoretical investigations. Oikos 2011, 120, 1557–1567. [Google Scholar]
- Wilcove, D.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. Bio-Science 1998, 48, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Handrinos, G.; Akriotis, T. The Birds of Greece; Christopher Helm: London, UK, 1997. [Google Scholar]
- Rolando, A.; Caprio, E.; Rinaldi, E.; Ellena, I. The impact of high-altitude ski-runs on alpine grassland bird communities. J. Appl. Ecol. 2006, 44, 210–219. [Google Scholar] [CrossRef]
- Barilani, M.; Bernard-Laurent, A.; Mucci, N.; Tabarroni, C.; Kark, S.; Garrido, J.A.P.; Randi, E. Hybridisation with introduced chukars (Alectoris chukar) threatens the gene pool integrity of native rock (A. graeca) and red-legged (A. rufa) partridge populations. Biol. Conserv. 2007, 137, 57–69. [Google Scholar] [CrossRef]
- Negri, A.; Pellegrino, I.; Mucci, N.; Randi, E.; Tizzani, P.; Meneguz, P.G.; Malacarne, G. Mitochondrial DNA and microsatellite markers evidence a different pattern of hybridization in red-legged partridge (Alectoris rufa) populations from NW Italy. Eur. J. Wildl. Res. 2013, 59, 407–419. [Google Scholar] [CrossRef] [Green Version]
- Gontero, C.; Fanelli, A.; Zanet, S.; Meneguz, P.G.; Tizzani, P. Exotic Species and Autochthonous Parasites: Trichostrongylus Retortaeformis in Eastern Cottontail. Life 2020, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Tizzani, P.; Andrade, D.; Molinar Min, A.R.; Peano, A.; Meneguz, P.G. Does the Introduction of Alien Species Represent a Sanitary Threat for Native Species? The Case of the Eastern Cottontail Sylvilagus floridanus in Italy. Life 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; Heath, M. Birds in Europe: Their Conservation Status; BirdLife Conservation Series; BirdLife: Cambridge, UK, 1994. [Google Scholar]
- Dobson, A.; Hudson, P. Regulation and Stability of a Free-Living Host-Parasite System: Trichostrongylus tenuis in Red Grouse. II. Population Models. J. Anim. Ecol. 1992, 61, 487–498. [Google Scholar] [CrossRef]
- Tompkins, D.M.; Dobson, A.P.; Arneberg, P.; Begon, M.E.; Cattadori, I.M.; Greenman, J.V.; Heesterbeek, J.A.P.; Hudson, P.J.; Newborn, D.; Pugliese, A.; et al. Parasites and host population dynamics. In The Ecology of Wildlife Diseases; Hudson, P., Rizzoli, A., Grenfell, B., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 45–62. [Google Scholar]
- Vallance, M. Faune Sauvage de France: Biologie, Habitats et Gestion; Christopher Helm: London, UK, 2007; p. 415. [Google Scholar]
- Juan-Sallés, C.; Garner, M. Avian Spirurids. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 9, pp. 471–480. [Google Scholar]
- Work, T.; Meteyer, C.; Cole, R. Mortality in Laysan Ducks (Anas laysanensis) by Emaciation Complicated by Echinuria uncinata on Laysan Island, Hawaii, 1993. J. Wildl. Dis. 2004, 40, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Dunham, N.; Peper, S.; Baxter, C.; Kendall, R. The Parasitic Eyeworm Oxyspirura petrowi as a Possible Cause of Decline in the Threatened Lesser Prairie-Chicken (Tympanuchus pallidicinctus). PLoS ONE 2014, 9, e108244. [Google Scholar] [CrossRef]
- Menezes, R.; Tortelly, R.; Gomes, D.; Pinto, R. Pathology and frequency of Cheilospirura hamulosa (Nematoda, Acuarioidea) in Galliformes hosts from backyard flocks. Avian Pathol. 2003, 32, 151–156. [Google Scholar] [CrossRef]
- Salam, S.; Mir, M.; Shahnaz, S.; Khan, R. Prevalence and the Associated Lesions of Cheilospirura (Acuaria) hamulosa in the Indigenous Chicken of Kashmir Valley, India. J. Parasitol. 2009, 95, 1436–1439. [Google Scholar] [CrossRef]
- Tizzani, P.; Fanelli, A.; Negri, E.; Silvano, F.; Menzano, A.; Molinar Min, A.; Meneguz, P.G. Haemoparasites in red-legged partridge (Alectoris rufa): First record of Haemoproteus sp. in Italy? J. Parasit. Dis. 2020, 44, 462–466. [Google Scholar] [CrossRef]
- Iacopelli, F.; Fanelli, A.; Tizzani, P.; Berriatua, E.; Prieto, P.; Martínez-Carrasco, C.; León, L.; Rossi, L.; Candela, M.G. Spatio-temporal patterns of sarcoptic mange in red deer and Iberian ibex in a multi-host natural park. Res. Vet. Sci. 2020, 128, 224–229. [Google Scholar] [CrossRef]
- Fanelli, A.; Tizzani, P.; Belleau, E. Gastrointestinal parasite infestation in the rock ptarmigan (Lagopus muta) in the French Alps and French Pyrenees based on long-term sampling (1987–2018). Parasitology 2020, 147, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, R.; Buermann, W.; Harrigan, R.; Bonneaud, C.; Loiseau, C.; Chasar, A.; Sepil, I.; Valkiūnas, G.; Iezhova, T.; Saatchi, S.; et al. Spatially explicit predictions of blood parasites in a widely distributed African rainforest bird. Proc. R. Soc. B Biol. Sci. 2011, 278, 1025–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samy, A.; Campbell, L.; Peterson, A. Leishmaniasis transmission: Distribution and coarse-resolution ecology of two vectors and two parasites in Egypt. Rev. Da Soc. Bras. De Med. Trop. 2014, 47, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchis-Monsonís, G.; Fanelli, A.; Tizzani, P.; Martínez-Carrasco, C. First epidemiological data on Spirocerca vulpis in the red fox: A parasite of clustered geographical distribution. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100338. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, P.; Morales-Castilla, I.; Park, A.; Huang, S.; Schmidt, J.P.; Stephens, P.R. Comparing methods for mapping global parasite diversity. Glob. Ecol. Biogeogr. 2020, 29, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Johnson, E.; Escobar, L.; Zambrana-Torrelio, C. An Ecological Framework for Modeling the Geography of Disease Transmission. Trends Ecol. Evol. 2019, 34, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Graham, C.H.P.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography (Cop) 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Mi, C.; Huettmann, F.; Guo, Y.; Han, X.; Wen, L. Why choose Random Forest to predict rare species distribution with few samples in large undersampled areas? Three Asian crane species models provide supporting evidence. PeerJ 2017, 5, e2849. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Del-Olmo, A.; Montero, F.; Fernndez, M.; Barrett, J.; Raga, J.A.; Kostadinova, A. Discrimination of fish populations using parasites: Random Forests on a predictable host-parasite system. Parasitology 2010, 137, 1833–1847. [Google Scholar] [CrossRef]
- MAFF. Manual of Veterinary Parasitological Laboratory Techniques; Her Majesty’s Stationary Office; MAFF: London, UK, 1986. [Google Scholar]
- Skrjabin, K.; Sobolev, A.; Ivashkin, V. Essentials of Nematodology. XIV. Spirurata of Animals and Man and the Diseases They Cause, Acuarioidea, Part 3; Akademii Nauk SSSR: Moscow, Russia, 1965. [Google Scholar]
- Ebrahimi, M.; Rouhani, S.; Mobedi, I.; Rostami, A.; Khazan, H.; Ahoo, M.B. Prevalence and Morphological Characterization of Cheilospirura hamulosa, Diesing, 1861 (Nematoda: Acuarioidea), from Partridges in Iran. J. Parasitol. Res. 2015, 2005, 569340. [Google Scholar]
- Rozsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Fick, S.; Hijmans, R. Worldclim 2, New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2017, 127, 1–15. [Google Scholar] [CrossRef] [Green Version]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation: Chicago, IL, USA, 2017. [Google Scholar]
- Kuhn, M. Caret: Classification and Regression Training. R Package Version 6.0-85. 2020. Available online: https://CRAN.R-project.org/package=caret (accessed on 25 September 2020).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Flach, P.; Hernández-Orallo, J. Proceedings of the 28th International Conference on Machine Learning. In A Coherent Interpretation of AUC as a Measure of Aggregated Classification Performance; ICML: Bellevue, WA, USA, 2011. [Google Scholar]
- Rizzoli, A.; Manfredi, M.T.; Rosso, F.; Rosà, R.; Cattadori, I.; Hudson, P. A survey to identify the important macroparasites of rock partridge (Alectoris graeca saxatilis) in Trentino, Italy. Parassitologia 1997, 39, 331–335. [Google Scholar] [PubMed]
- Formenti, N.; Viganò, R.; Ferrari, N.; Cerutti, M.C.; Lanfranchi, P. Helminth community of an alpine rock partridge (Alectoris græca) population in demographic crash. In Wildlife Disease Association (WDA) & European Wildlife Disease Association (EWDA) Conference; Wildlife Disease Association: Lion, France, 2012; p. 1. [Google Scholar]
- Fanelli, A.; Menardi, G.; Chiodo, M.; Giordano, O.; Ficetto, G.; Bessone, M.; Lasagna, A.; Carpignano, M.G.; Molinar, M.A.; Gugiatti, A.; et al. Gastroenteric parasite of wild Galliformes in the Italian Alps: Implication for conservation management. Parasitology 2020, 147, 471–477. [Google Scholar] [CrossRef]
- De Marco, M.A.; Barchetti, A.; Guberti, V. Gastro-intestinal Helminths of three Galliformes species in the Italian Alps. Sel. Vet. 1999, 2001, 699–704. [Google Scholar]
- Eslami, A.; Ghaemi, P.; Rahbari, S. Parasitic infections of free -range chickens from Golestan Province, Iran. Iran. J. Parasitol. 2009, 4, 10–14. [Google Scholar]
- Gomes, D.; Menezes, R.; Vicente, J.; Lanfredi, R.M.; Pinto, R.M. New morphological data on Cheilospirura hamulosa (Nematoda, Acuarioidea) by means of bright-field and scanning electron microscopy. Parasitol. Res. 2004, 92, 225–231. [Google Scholar] [CrossRef]
- Atkinson, C.; Thomas, N.; Hunter, D. Parasitic Diseases of Wild Birds; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Poulin, R. Helminth growth in vertebrate hosts: Does host sex matter? Int. J. Parasitol. 1996, 26, 1311–1315. [Google Scholar] [CrossRef]
- Poulin, R. Sexual inequalities in helminth infections: A cost of being a male? Am. Nat. 1996, 147, 287–295. [Google Scholar] [CrossRef]
- Lopez, J. Parasite prevalence and the size of host populations: An experimental test. J. Parasitol. 2005, 91, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hudson, P.J.; Newborn, D.; Dobson, A.P. Regulation and stability of a free-living host-parasite system: Tricostrongylus tenuis in red grouse. I. Monitoring and parasite reduction experiments. J. Anim. Ecol. 1992, 61, 477–486. [Google Scholar] [CrossRef]
- Short, E.E.; Caminade, C.; Thomas, B.N. Climate Change Contribution to the Emergence or Re-Emergence of Parasitic Diseases. Infect. Dis. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Tripet, F.; Christe, P.; Moller, A. The importance of host spatial distribution for parasite specialization and speciation: A comparative study of bird fleas (Siphonaptera: Ceratophyllidae). J. Anim. Ecol. 2002, 71, 735–748. [Google Scholar] [CrossRef]
- Evans, J.; Murphy, M.; Holden, Z.; Cushman, S. Modeling Species Distribution and Change Using Random forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Springer: New York, NY, USA, 2001; pp. 139–159. [Google Scholar]
- Koch, B.; Edwards, P.; Blanckenhorn, W.; Walter, T.; Hofer, G. Shrub Encroachment Affects the Diversity of Plants, Butterflies, and Grasshoppers on Two Swiss Subalpine Pastures. Arct. Antarct. Alp. Res. 2015, 47, 345–357. [Google Scholar] [CrossRef] [Green Version]





| Variable | Description |
|---|---|
| BIO1 | Annual Mean Temperature |
| BIO4 | Temperature Seasonality (standard deviation × 100) |
| BIO8 | Mean Temperature of Wettest Quarter |
| BIO10 | Mean Temperature of Warmest Quarter |
| BIO11 | Mean Temperature of Coldest Quarter |
| BIO12 | Annual Precipitation |
| BIO15 | Precipitation Seasonality (Coefficient of Variation) |
| BIO17 | Precipitation of Driest Quarter |
| BIO18 | Precipitation of Warmest Quarter |
| BIO19 | Precipitation of Coldest Quarter |
| DEM | Digital elevation model (DEM) describing the altitude for each pixel |
| Latitude | Raster describing the latitude in UTM coordinates of each pixel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanelli, A.; Tizzani, P.; Ferroglio, E.; Belleau, E. Cheilospirura hamulosa in the Rock Partridge (Alectoris graeca saxatilis): Epidemiological Patterns and Prediction of Parasite Distribution in France. Diversity 2020, 12, 484. https://doi.org/10.3390/d12120484
Fanelli A, Tizzani P, Ferroglio E, Belleau E. Cheilospirura hamulosa in the Rock Partridge (Alectoris graeca saxatilis): Epidemiological Patterns and Prediction of Parasite Distribution in France. Diversity. 2020; 12(12):484. https://doi.org/10.3390/d12120484
Chicago/Turabian StyleFanelli, Angela, Paolo Tizzani, Ezio Ferroglio, and Eric Belleau. 2020. "Cheilospirura hamulosa in the Rock Partridge (Alectoris graeca saxatilis): Epidemiological Patterns and Prediction of Parasite Distribution in France" Diversity 12, no. 12: 484. https://doi.org/10.3390/d12120484
APA StyleFanelli, A., Tizzani, P., Ferroglio, E., & Belleau, E. (2020). Cheilospirura hamulosa in the Rock Partridge (Alectoris graeca saxatilis): Epidemiological Patterns and Prediction of Parasite Distribution in France. Diversity, 12(12), 484. https://doi.org/10.3390/d12120484