Insights into the Fungal Community and Functional Roles of Pepper Rhizosphere Soil under Plastic Shed Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. DNA Extraction and High-Throughput Sequencing
2.3. FUNGuild Analysis
2.4. Data Analysis and Statistics
3. Results and Discussion
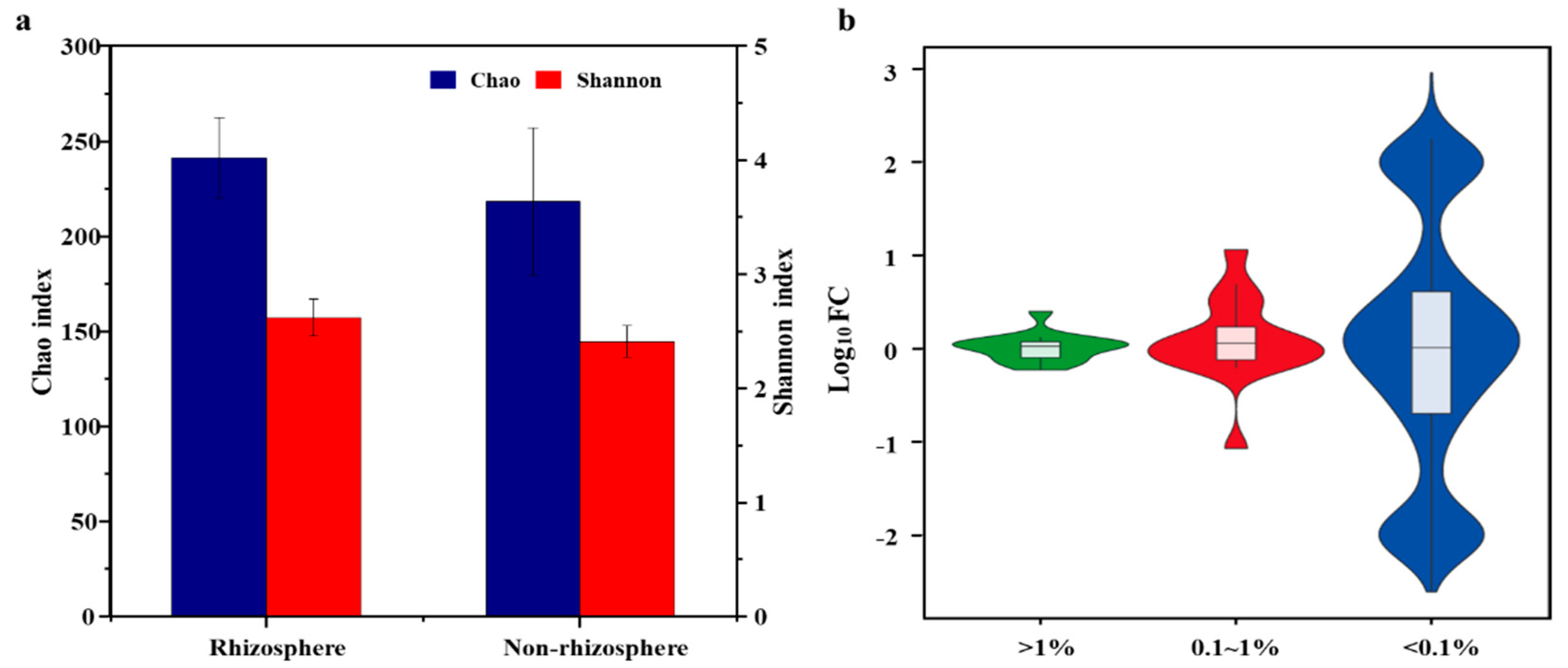
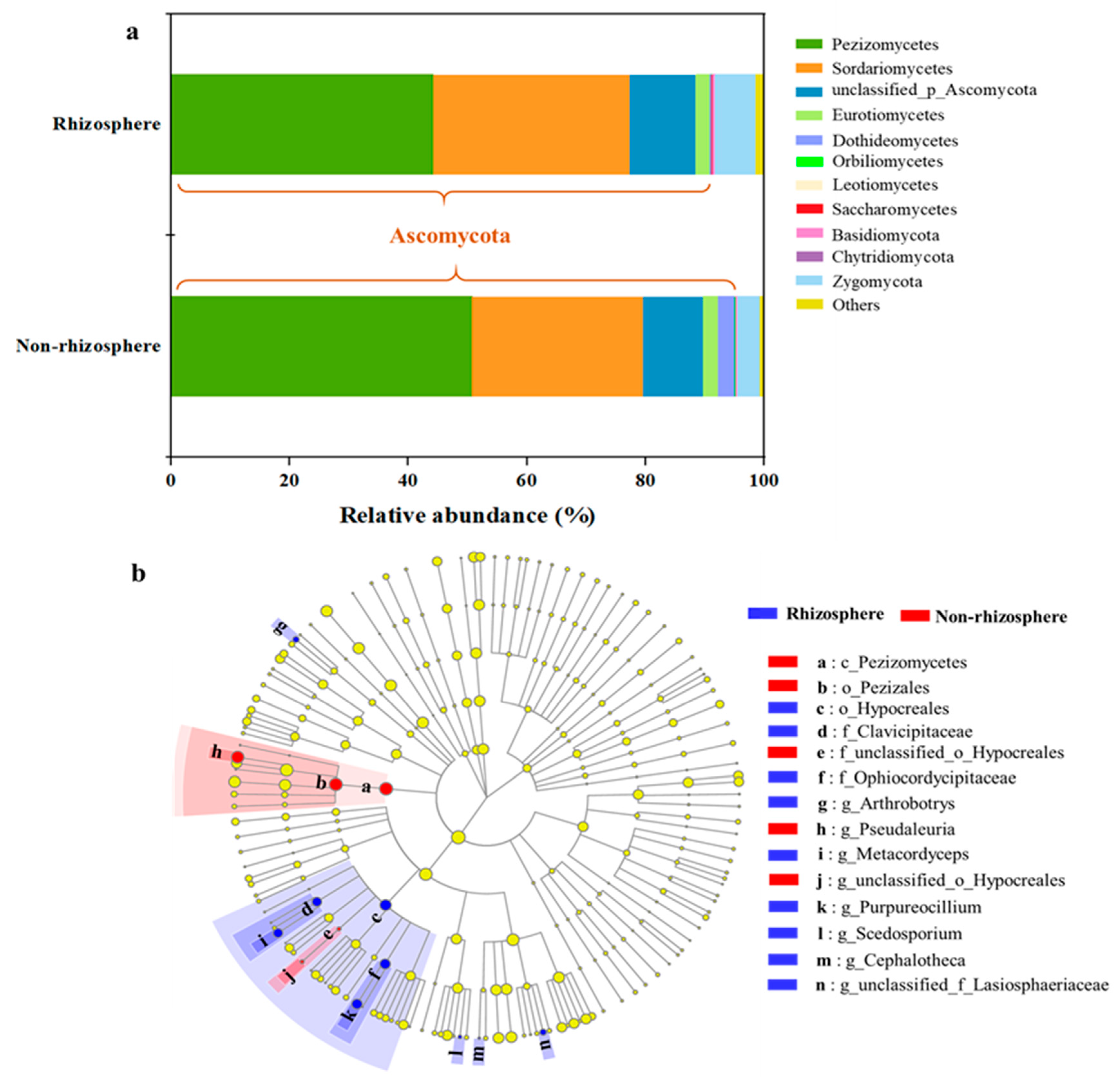
3.1. Shifts in Fungal Community Structure in Soil
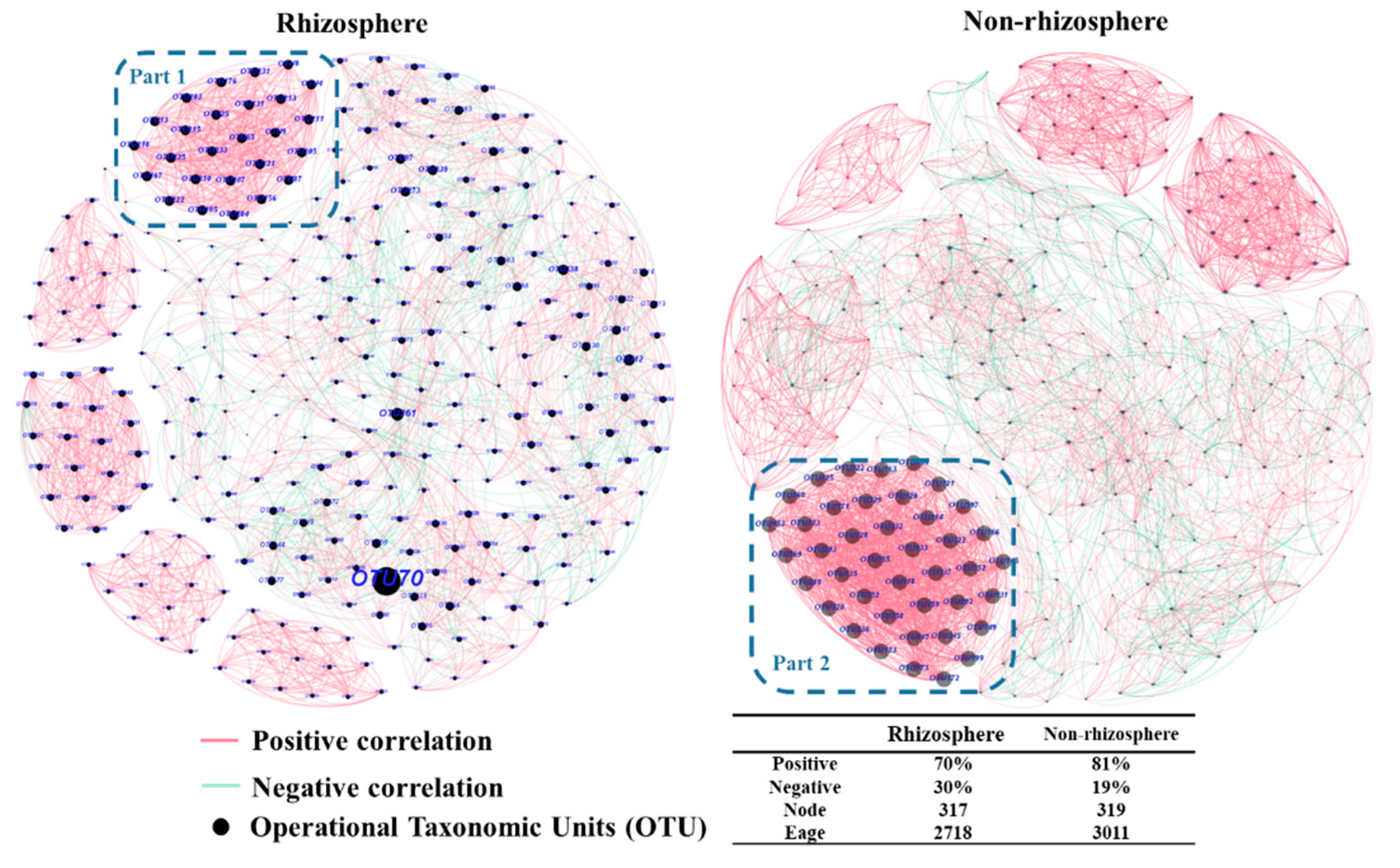
3.2. Interaction of Fungal Communities
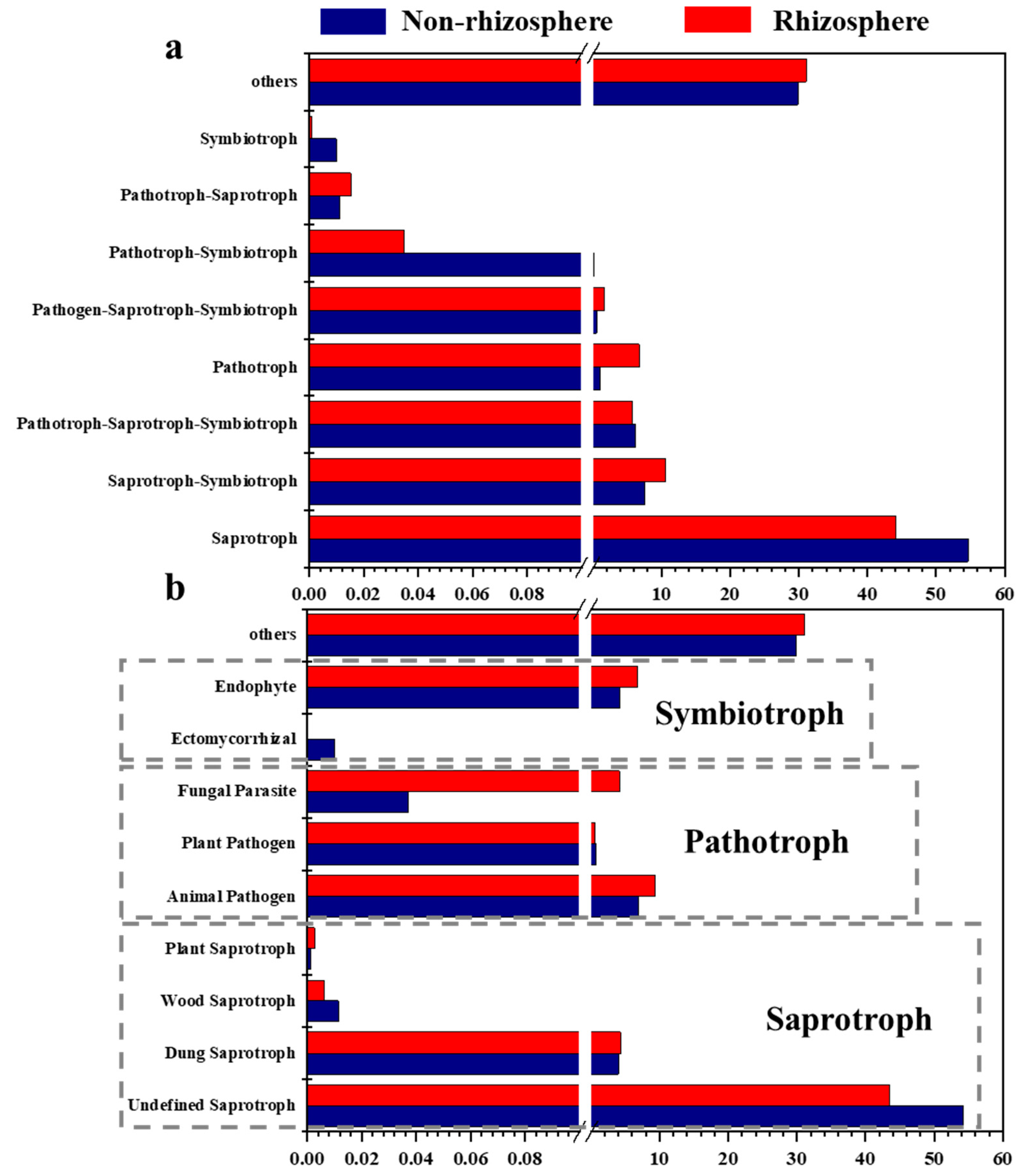
3.3. Shifts in the Fungal Functional Role in Soil
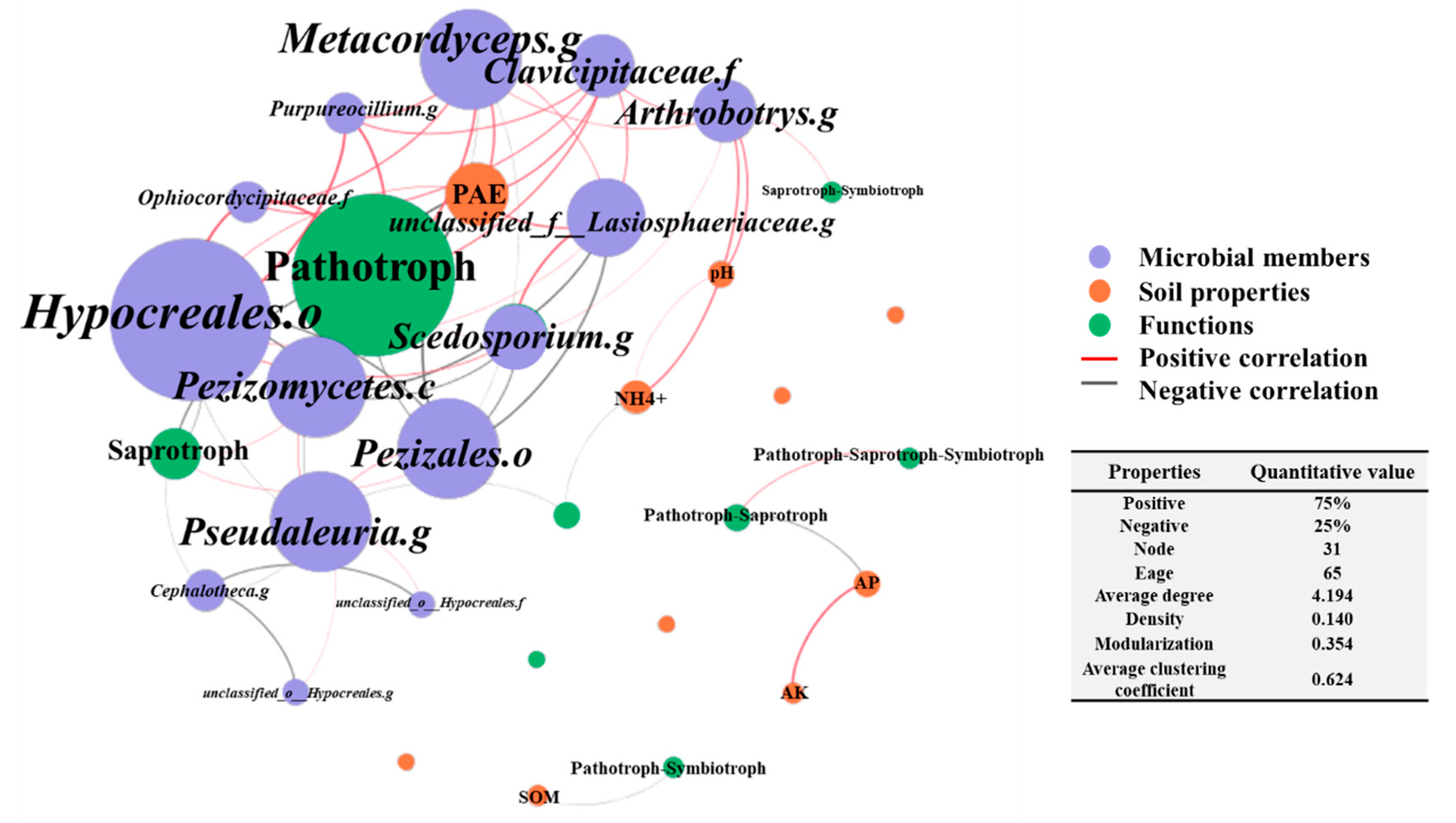
3.4. Co-Occurrence Network among the Fungal Community, Functional Roles and Soil Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, J.; Wu, X.; Wang, Y.; Meyerson, L.A.; Gu, B.J.; Min, Y.; Xue, H.; Peng, C.H.; Ge, Y. Does growing vegetables in plastic greenhouses enhance regional ecosystem services beyond the food supply? Front. Ecol. Environ. 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Sun, J.T.; Pan, L.L.; Li, Z.H.; Zeng, Q.T.; Wang, L.W.; Zhu, L.Z. Comparison of greenhouse and open field cultivations across China: Soil characteristics, contamination and microbial diversity. Environ. Pollut. 2018, 243, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Yang, S.; Li, X.X.; He, L.F.; Zhu, J.M.; Mu, W.; Liu, F. Residue determination of pyraclostrobin, picoxystrobin and its metabolite in pepper fruit via UPLC-MS/MS under open field conditions. Ecotox. Environ. Safe 2019, 182, e109445. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Liu, B.; Wu, G.; Sun, Y.X.; Guo, X.S.; Jin, Z.H.; Xu, W.N.; Zhao, Y.Z.; Zhang, F.S.; Zou, C.Q.; et al. Environmental costs and mitigation potential in plastic-greenhouse pepper production system in China: A life cycle assessment. Agric. Syst. 2018, 167, 186–194. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Li, X.N.; Bian, Y.R.; Wang, F.; Yang, X.L.; Gu, C.G.; Jiang, X. Long-Term Plastic Greenhouse Cultivation Changes Soil Microbial Community Structures: A Case Study. J. Agric. Food. Chem. 2018, 66, 8941–8948. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Y.; Zhang, Y.X.; Huang, B.; Teng, Y. Soil environmental quality in greenhouse vegetable production systems in eastern China: Current status and management strategies. Chemosphere 2017, 170, 183–195. [Google Scholar] [CrossRef]
- Ning, Q.; Chen, L.; Jia, Z.J.; Zhang, C.Z.; Ma, D.H.; Li, F.; Zhang, J.B.; Li, D.M.; Han, X.R.; Cai, Z.J.; et al. Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agric. Ecosyst. Environ. 2020, 293, e106837. [Google Scholar] [CrossRef]
- Van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A thready affair: Linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef]
- Martinez-Diz, M.D.; Andres-Sodupe, M.; Bujanda, R.; Diaz-Losada, E.; Eichmeier, A.; Gramaje, D. Soil-plant compartments affect fungal microbiome diversity and composition in grapevine. Fungal Ecol. 2019, 41, 234–244. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Xing, J.J.; Gu, H.P.; Wang, H.Z.; Wu, J.J.; Xu, J.M.; Brookes, P.C. Development of microbial community structure in vegetable-growing soils from open-field to plastic-greenhouse cultivation based on the PLFA analysis. J. Soils Sediments 2016, 16, 2041–2049. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.U.; Awan, M.F.; Fatima, K.; Tahir, M.S.; Ali, Q.; Rashid, B.; Rao, A.Q.; Nasir, I.A.; Husnain, T. Phytophthora capsici on chilli pepper (Capsicum annuum L.) and its management through genetic and bio-control: A review. Zemdirb. Agric. 2016, 103, 419–430. [Google Scholar] [CrossRef]
- Sergaki, C.; Lagunas, B.; Lidbury, I.; Gifford, M.L.; Schafer, P. Challenges and approaches in microbiome research: From fundamental to applied. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Li, X.G.; Ding, C.F.; Hua, K.; Zhang, T.L.; Zhang, Y.N.; Zhao, L.; Yang, Y.R.; Liu, J.G.; Wang, X.X. Soil sickness of peanuts is attributable to modifications in soil microbes induced by peanut root exudates rather than to direct allelopathy. Soil Biol. Biochem. 2014, 78, 149–159. [Google Scholar] [CrossRef]
- Li, S.L.; Xu, C.; Wang, J.; Guo, B.; Yang, L.; Chen, J.N.; Ding, W. Cinnamic, myristic and fumaric acids in tobacco root exudates induce the infection of plants by Ralstonia solanacearum. Plant Soil 2017, 412, 381–395. [Google Scholar] [CrossRef]
- Li, Z.G.; Zu, C.; Wang, C.; Yang, J.F.; Yu, H.; Wu, H.S. Different responses of rhizosphere and non-rhizosphere soil microbial communities to consecutive Piper nigrum L. monoculture. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, L.; Yang, J.F.; Duan, Y.M.; Luo, Y.; Taherzadeh, M.J.; Li, Y.F.; Li, H.K.; Awasthi, M.K.; Zhao, Z.Y. The diversity of microbial community and function varied in response to different agricultural residues composting. Sci. Total Environ. 2020, 715, e136983. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Feng, H.J.; Wang, S.Y.; Gao, Z.D.; Wang, Z.K.; Ren, X.Q.; Hu, S.W.; Pan, H. Effect of land use on the composition of bacterial and fungal communities in saline-sodic soils. Land Degrad. Dev. 2019, 30, 1851–1860. [Google Scholar] [CrossRef]
- Lian, T.X.; Mu, Y.H.; Jin, J.; Ma, Q.B.; Cheng, Y.B.; Cai, Z.D.; Nian, H. Impact of intercropping on the coupling between soil microbial community structure, activity, and nutrient-use efficiencies. Peer J. 2019, 7, e6412. [Google Scholar] [CrossRef] [PubMed]
- Skaltsas, D.N.; Badotti, F.; Vaz, A.B.M.; da Silva, F.F.; Gazis, R.; Wurdack, K.; Castlebury, L.; Goes-Neto, A.; Chaverri, P. Exploration of stem endophytic communities revealed developmental stage as one of the drivers of fungal endophytic community assemblages in two Amazonian hardwood genera. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.A.; Lei, X.M.; Zhao, L.X.; Brookes, P.C.; Wang, F.; Chen, C.R.; Yang, W.H.; Xing, S.H. Fungal communities and functions response to long-term fertilization in paddy soils. Appl. Soil Ecol. 2018, 130, 251–258. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.J.; Han, X.Z.; Song, F.B.; Zhang, Z.M.; Yan, J.; Xu, Y.L. A comprehensive analysis of the response of the fungal community structure to long-term continuous cropping in three typical upland crops. J. Integr. Agric. 2020, 19, 866–880. [Google Scholar] [CrossRef]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated control of Meloidogyne incognita in tomatoes using fluopyram and Purpureocillium lilacinum strain 251. Crop Prot. 2019, 124, e104874. [Google Scholar] [CrossRef]
- Moreno-Salazar, R.; Sanchez-Garcia, I.; Chan-Cupul, W.; Ruiz-Sanchez, E.; Hernandez-Ortega, H.A.; Pineda-Lucatero, J.; Figueroa-Chavez, D. Plant growth, foliar nutritional content and fruit yield of Capsicum chinense biofertilized with Purpureocillium lilacinum under greenhouse conditions. Sci. Hortic. 2020, 261, e108950. [Google Scholar] [CrossRef]
- Strom, N.; Hu, W.M.; Haarith, D.; Chen, S.Y.; Bushley, K. Corn and soybean host root endophytic fungi with toxicity toward the soybean cyst nematode. Phytopathology 2020, 110, 603–614. [Google Scholar] [CrossRef]
- Nimnoi, P.; Ruanpanun, P. Suppression of root-knot nematode and plant growth promotion of chili (Capsicum flutescens L.) using co-inoculation of Streptomyces spp. Biol. Control 2020, 145, e104244. [Google Scholar] [CrossRef]
- Gine, A.; Carrasquilla, M.; Martinez-Alonso, M.; Gaju, N.; Sorribas, F.J. Characterization of soil suppressiveness to root-knot nematodes in organic horticulture in plastic greenhouse. Front. Plant Sci. 2016, 7, e00164. [Google Scholar] [CrossRef]
- Mouhajir, A.; Poirier, W.; Angebault, C.; Rahal, E.; Bouabid, R.; Bougnoux, M.E.; Kobi, A.; Zouhair, R.; Bouchara, J.P.; Giraud, S. Scedosporium species in soils from various biomes in Northwestern Morocco. PLoS ONE 2020, 15, e0228897. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, X.; Yao, S.; Yang, X.; Jiang, X. Correlations between soil metabolomics and bacterial community structures in the pepper rhizosphere under plastic greenhouse cultivation. Sci. Total Environ. 2020, 728, 138439. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.J.; Liu, J.; Zhang, J.; Li, D.M.; Xu, C.X.; Kuzyakov, Y. Divergence in fungal abundance and community structure between soils under long-term mineral and organic fertilization. Soil Tillage Res. 2020, 196, 104491. [Google Scholar] [CrossRef]
- Jiao, S.; Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Chang. Biol. 2020. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.N.; Xu, M.; Jiao, W.; Bian, Y.R.; Yang, X.L.; Gu, C.G.; Wang, F.; Jiang, X. Does biochar induce similar successions of microbial community structures among different soils? Bull. Environ. Contam. Toxicol. 2019, 103, 642–650. [Google Scholar] [CrossRef]
- Pedros-Alio, C. The rare bacterial biosphere. Annu. Rev. Mar. Sci. 2012, 4, 449–466. [Google Scholar] [CrossRef]
- Kong, X.; Jin, D.C.; Wang, X.X.; Zhang, F.S.; Duan, G.L.; Liu, H.J.; Jia, M.H.; Deng, Y. Dibutyl phthalate contamination remolded the fungal community in agro-environmental system. Chemosphere 2019, 215, 189–198. [Google Scholar] [CrossRef]
- Liu, L.L.; Huang, X.Q.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Characterizing the key agents in a disease-suppressed soil managed by reductive soil disinfestation. Appl. Environ. Microbiol. 2019, 85, 15. [Google Scholar] [CrossRef]
- Kepler, R.M.; Maul, J.E.; Rehner, S.A. Managing the plant microbiome for biocontrol fungi: Examples from Hypocreales. Curr. Opin. Microbiol. 2017, 37, 48–53. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph: Saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Chen, W.Q.; Wang, J.Y.; Meng, Z.X.; Xu, R.; Chen, J.; Zhang, Y.J.; Hu, T.M. Fertility-related interplay between fungal guilds underlies plant richness-productivity relationships in natural grasslands. New Phytol. 2020, 226, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Kolarikova, Z.; Kohout, P.; Kruger, C.; Janouskova, M.; Mrnka, L.; Rydlova, J. Root-associated fungal communities along a primary succession on a mine spoil: Distinct ecological guilds assemble differently. Soil Biol. Biochem. 2017, 113, 143–152. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Cajthaml, T.; Polme, S.; Hiiesalu, I.; Anslan, S.; Harend, H.; Buegger, F.; Pritsch, K.; Koricheva, J.; et al. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J. 2016, 10, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, Y. Potential allelopathic effects of allelochemicals in aqueous extracts of leaves and root exudates of Capsicum annuum on vegetable crops. Allelopath. J. 2015, 35, 11–22. [Google Scholar]
- Floc’h, J.B.; Hamel, C.; Harker, K.N.; St-Arnaud, M. Fungal communities of the canola rhizosphere: Keystone species and substantial between-year variation of the rhizosphere microbiome. Microb. Ecol. 2020. [Google Scholar] [CrossRef]
- Kokalis-Burelle, N.; McSorley, R.; Wang, K.H.; Saha, S.K.; McGovern, R.J. Rhizosphere microorganisms affected by soil solarization and cover cropping in Capsicum annuum and Phaseolus lunatus agroecosystems. Appl. Soil Ecol. 2017, 119, 64–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Li, X.; Cheng, H.; Sun, K.; Jiang, X.; Song, Y. Insights into the Fungal Community and Functional Roles of Pepper Rhizosphere Soil under Plastic Shed Cultivation. Diversity 2020, 12, 432. https://doi.org/10.3390/d12110432
Yao S, Li X, Cheng H, Sun K, Jiang X, Song Y. Insights into the Fungal Community and Functional Roles of Pepper Rhizosphere Soil under Plastic Shed Cultivation. Diversity. 2020; 12(11):432. https://doi.org/10.3390/d12110432
Chicago/Turabian StyleYao, Shi, Xiaona Li, Hu Cheng, Kaining Sun, Xin Jiang, and Yang Song. 2020. "Insights into the Fungal Community and Functional Roles of Pepper Rhizosphere Soil under Plastic Shed Cultivation" Diversity 12, no. 11: 432. https://doi.org/10.3390/d12110432
APA StyleYao, S., Li, X., Cheng, H., Sun, K., Jiang, X., & Song, Y. (2020). Insights into the Fungal Community and Functional Roles of Pepper Rhizosphere Soil under Plastic Shed Cultivation. Diversity, 12(11), 432. https://doi.org/10.3390/d12110432