Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata)
Abstract
1. Introduction
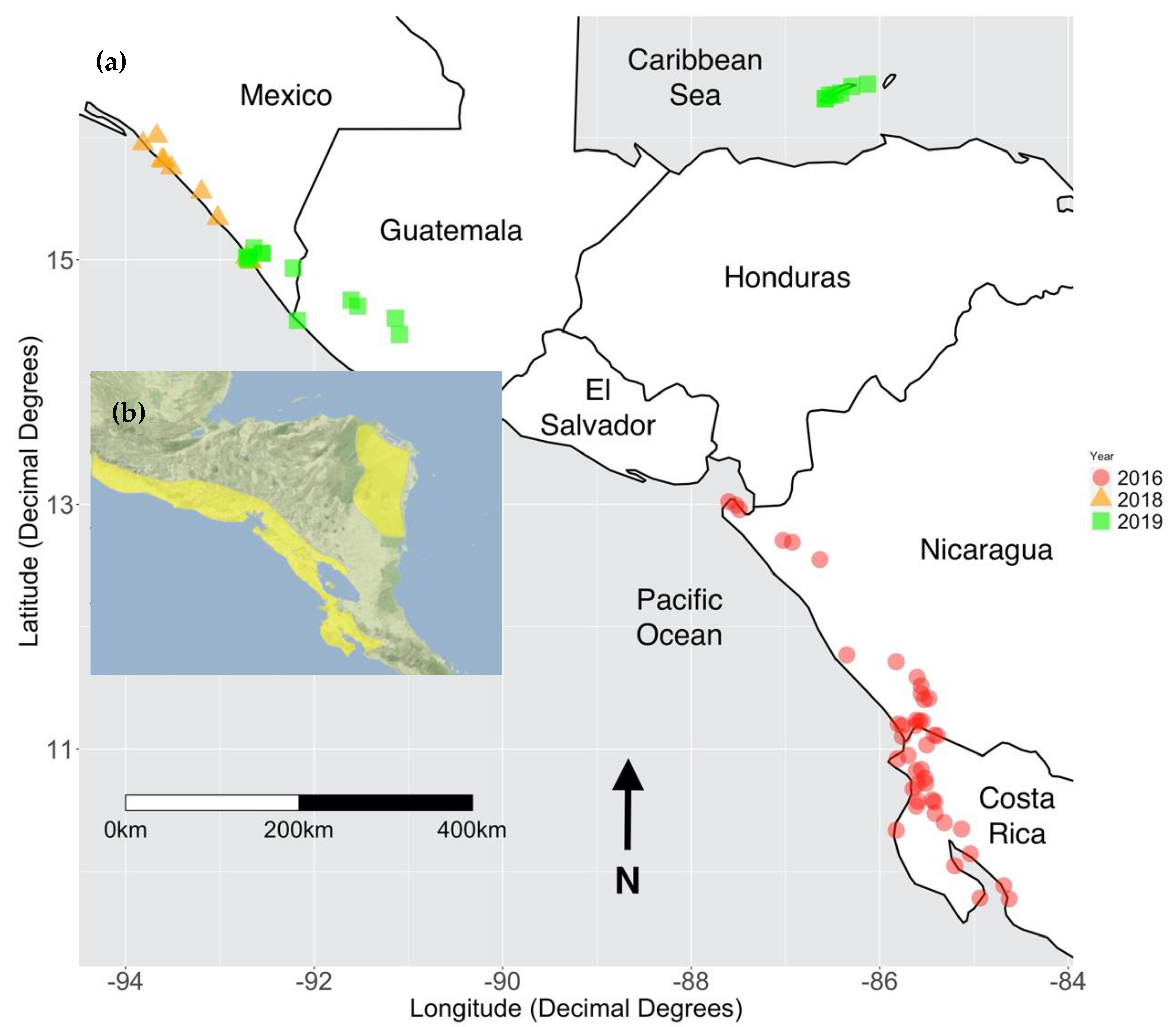
2. Materials and Methods
3. Results
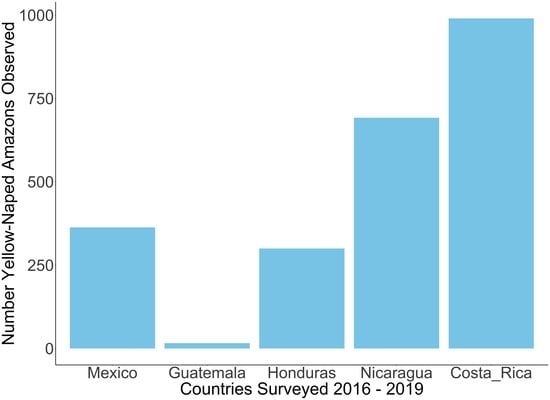
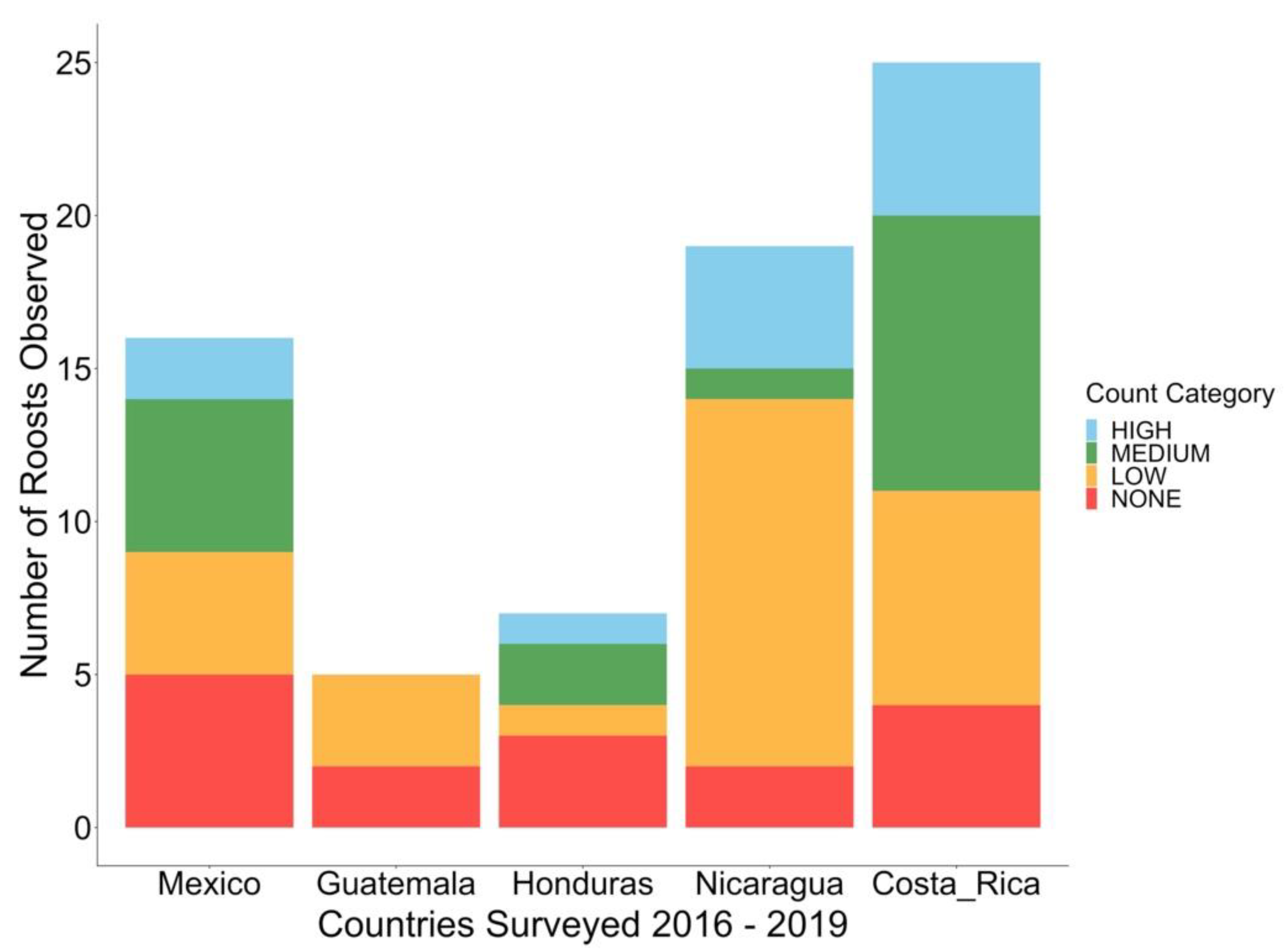
3.1. Roost Count Results
3.2. Estimated Differences between Traditional Roost Counts and eBird Database Reports
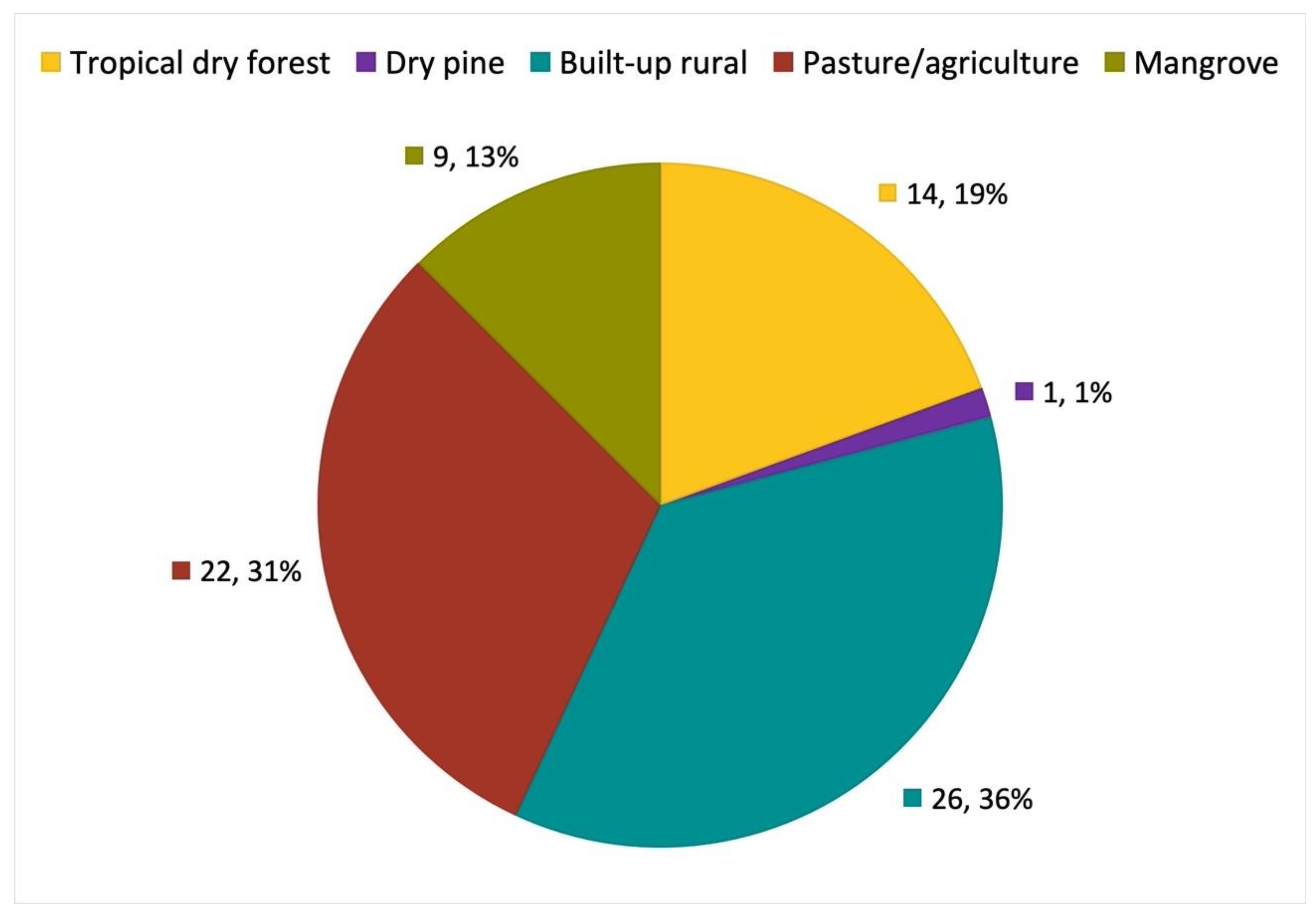
3.3. Roost Characteristics
4. Discussion
4.1. Range-Wide Population Estimates for the Yellow-Naped Amazon
4.2. Threats to Populations
4.3. The Utility of Citizen Science Approaches to Monitoring Populations
4.4. Implications for Conservation and Management
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Sabogal, C. Regeneration of tropical dry forests in Central-America, with examples from Nicaragua. J. Veg. Sci. 1992, 3, 407–416. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Harvey, C.A.; Martínez-Ramos, M.; Balvanera, P.; Stoner, K.E.; Schondube, J.E.; Avila Cabadilla, L.D.; Flores-Hidalgo, M. Seasonally dry tropical forest biodiversity and conservation value in agricultural landscapes of Mesoamerica. In Seasonally Dry Tropical Forests; Dirzo, R., Young, H.S., Mooney, H.A., Ceballos, G., Eds.; Island Press: Washington, DC, USA, 2011; pp. 195–219. [Google Scholar]
- Visco, D.M.; Michel, N.L.; Boyle, W.A.; Sigel, B.J.; Woltmann, S.; Sherry, T.W. Patterns and causes of understory bird declines in human-disturbed tropical forest landscapes: A case study from Central America. Biol. Conserv. 2015, 191, 117–129. [Google Scholar] [CrossRef]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.C.; Aguilar, J.; Aleman-Zelaya, U.; Aramburu, R.M.; Ariash, A.A.; McNab, R.B.; Balsby, T.J.S.; et al. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef]
- Wright, T.F.; Toft, C.A.; Enkerlin-Hoeflich, E.; Gonzalez-Elizondo, J.; Albornoz, M.; Rodriguez-Ferraro, A.; Rojas-Suarez, F.; Sanz, V.; Trujillo, A.; Beissinger, S.R.; et al. Nest poaching in neotropical parrots. Conserv. Biol. 2001, 15, 710–720. [Google Scholar] [CrossRef]
- BirdLife International. Psittaciformes assessment. In The IUCN Red List of Threatened Species; The International Union for Conservation of Nature: Cambridge, UK, 2019. [Google Scholar]
- Denes, F.V.; Tella, J.L.; Beissinger, S.R. Revisiting methods for estimating parrot abundance and population size. Emu Austral Ornithol. 2018, 118, 67–79. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P. Direct mortality of birds from anthropogenic causes. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 99–120. [Google Scholar] [CrossRef]
- BirdLife International. Amazona auropalliata. The IUCN Red List of Threatened Species 2017; E.T22686342A118961453; The International Union for Conservation of Nature: Cambridge, UK, 2017. [Google Scholar]
- Wright, T.F.; Lewis, T.C.; Lezama-Lopez, M.; Smith-Vidaurre, G.; Dahlin, C.R. Yellow-naped Amazon Amazona auropalliata populations are markedly low and rapidly declining in Costa Rica and Nicaragua. Bird Conserv. Int. 2019, 29, 291–307. [Google Scholar] [CrossRef]
- Dahlin, C.R.; Blake, C.; Rising, J.; Wright, T.F. Long-term monitoring of Yellow-naped Amazons (Amazona auropalliata) in Costa Rica: Breeding biology, duetting, and the negative impact of poaching. J. Field Ornithol. 2018, 89, 1–10. [Google Scholar] [CrossRef]
- Wright, T.F.; Dahlin, C.R. Vocal dialects in parrots: Patterns and processes of cultural evolution. Emu 2018, 118, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.F. Regional dialects in the contact call of a parrot. Proc. R. Soc. B Biol. Sci. 1996, 263, 867–872. [Google Scholar] [CrossRef]
- Drews, C. The state of wild animals in the minds and households of a neotropical society: The Costa Rican case study. In The State of the Animals II: 2003; Rowan, A.N., Salem, D.J., Eds.; Humane Society Press: Washington, DC, USA, 2003; pp. 193–205. [Google Scholar]
- Henry, E.H.; Haddad, N.M.; Wilson, J.; Hughes, P.; Gardner, B. Point-count methods to monitor butterfly populations when traditional methods fail: A case study with Miami blue butterfly. J. Insect Conserv. 2015, 19, 519–529. [Google Scholar] [CrossRef]
- Matuzak, G.D.; Brightsmith, D.J. Roosting of Yellow-naped Parrots in Costa Rica: Estimating the size and recruitment of threatened populations. J. Field Ornithol. 2007, 78, 159–169. [Google Scholar] [CrossRef]
- De Moura, L.N.; Vielliard, J.M.E.; Da Silva, M.L. Seasonal fluctuation of the Orange-winged Amazon at a roosting site in Amazonia. (Report). Wilson J. Ornithol. 2010, 122, 88. [Google Scholar] [CrossRef]
- Joyner, L. Guide to Multiple Point Fixed Transects in Parrot Monitoring; One Earth Conservation: New York, NY, USA, 2020. [Google Scholar]
- BirdLife International and Handbook of the Birds of the World. Bird Species Distribution Maps of the World. Version 2019.1. 2019. Available online: http://datazone.birdlife.org/species/requestdis (accessed on 10 June 2020).
- Horns, J.J.; Adler, F.R.; Sekercioglu, C.H. Using opportunistic citizen science data to estimate avian population trends. Biol. Conserv. 2018, 221, 151–159. [Google Scholar] [CrossRef]
- Kamp, J.; Oppel, S.; Heldbjerg, H.; Nyegaard, T.; Donald, P.F. Unstructured citizen science data fail to detect long-term population declines of common birds in Denmark. Divers. Distrib. 2016, 22, 1024–1035. [Google Scholar] [CrossRef]
- Walker, J.; Taylor, P.D. Using eBird data to model population change of migratory bird species. Avian Conserv. Ecol. 2017, 12. [Google Scholar] [CrossRef]
- Roldán-Clará, B.; López-Medellín, X.; Espejel, I.; Arellano, E. Literature review of the use of birds as pets in Latin-America, with a detailed perspective on Mexico. Ethnobiol. Conserv. 2014, 3. [Google Scholar] [CrossRef]
- Harvey, C.A.; Alpízar, F.; Chacón, M.; Madrigal, R. Assessing Linkages between Agriculture and Biodiversity in Central America: Historical Overview and Future Perspectives; The Nature Conservancy: San José, Costa Rica, 2004. [Google Scholar]
- Aide, T.M.; Clark, M.L.; Grau, H.R.; Lopez-Carr, D.; Levy, M.A.; Redo, D.; Bonilla-Moheno, M.; Riner, G.; Andrade-Nunez, M.J.; Muniz, M. Deforestation and Reforestation of Latin America and the Caribbean (2001–2010). Biotropica 2013, 45, 262–271. [Google Scholar] [CrossRef]
- Tryjanowski, P.; Kosicki, J.Z.; Hromada, M.; Mikula, P. The emergence of tolerance of human disturbance in Neotropical birds. J. Trop. Ecol. 2020, 36, 1–5. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen Science as an Ecological Research Tool: Challenges and Benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Aycrigg, J.L.; Barry, J.H.; Bonney, R.E.; Bruns, N.; Cooper, C.B.; Damoulas, T.; Dhondt, A.A.; Dietterich, T.; Farnsworth, A.; et al. The eBird enterprise: An integrated approach to development and application of citizen science. Biol. Conserv. 2014, 169, 31–40. [Google Scholar] [CrossRef]
- Robinson, O.J.; Ruiz-Gutierrez, V.; Fink, D.; Meese, R.J.; Holyoak, M.; Cooch, E.G. Using citizen science data in integrated population models to inform conservation. Biol. Conserv. 2018, 227, 361–368. [Google Scholar] [CrossRef]
- Pimm, S.L.; Alibhai, S.; Bergl, R.; Dehgan, A.; Giri, C.; Jewell, Z.; Joppa, L.; Kays, R.; Loarie, S. Emerging technologies to conserve biodiversity. Trends Ecol. Evol. 2015, 30, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Berglund, P.-A.; Hentati-Sundberg, J. Arctic Seabirds Breeding in the African-Eurasian Waterbird Agreement (AEWA) Area: Status and Trends 2014; Conservation of Arctic Flora and Fauna: Akureyri, Iceland, 2015. [Google Scholar]
- Lourenço, P.M.; Alonso, H.; Alves, J.A.; Carvalho, A.T.; Catry, T.; Costa, H.; Costa, J.S.; Dias, M.P.; Encarnação, V.; Fernandes, P.; et al. Monitoring waterbird populations in the Tejo estuary, Portugal: Report for the decade 2007–2016. Airo 2018, 25, 3–31. [Google Scholar]
- Hesse, A.J.; Duffield, G.E. The status and conservation of the Blue-Throated Macaw Ara glaucogularis. Bird Conserv. Int. 2000, 10, 255–275. [Google Scholar] [CrossRef]
- Herzog, S.K.; Maillard, Z.O.; Boorsma, T.; Sanchez-Avila, G. First systematic sampling approach to estimating the global population size of the Critically Endangered Blue-throated Macaw Ara glaucogularis. Bird Conserv. Int. 2020, 1–19. [Google Scholar] [CrossRef]
- Berkunsky, I.; Diaz Luque, J.A.; Kacoliris, F.P.; Daniele, G.; Milpacher, S.; Gilardi, J.D.; Martin, S. Blue-throated Macaw—10 Years. PsittaScene 2012, 24, 3–5. [Google Scholar]
- Vaughan, C.; Nemeth, N.; Marineros, L. Ecology and management of natural and artificial scarlet macaw (Are macao) nest cavities in Costa Rica. Ornitol. Neotrop. 2003, 14, 381–396. [Google Scholar]
- Ferreira Pires, S. The Illegal Parrot Trade in the Neo-Tropics. Ph.D. Thesis, Rutgers University, Newark, NJ, USA, 2012. [Google Scholar]
- Pires, S.F. The illegal parrot trade: A literature review. Glob. Crime 2012, 13, 176–190. [Google Scholar] [CrossRef]
| Country | Year | Site Name | Latitude | Longitude | Roost Behavior | Roost Observed | Roost Count | Roost Estimate | eBird Count | Habitat Type |
|---|---|---|---|---|---|---|---|---|---|---|
| Mexico | 2018 | Aztlan Site 3 | 14°58.841′ | 92°40.158′ | Yes | Yes | 33 | - | 24 | Pasture/Agriculture |
| Mexico | 2018 | Aztlan Site 2 | 14°59.596′ | 92°40.731′ | Yes | No | 38 | - | 24 | Pasture/Agriculture |
| Mexico | 2018 | Aztlan Site 1 | 15°0.045′ | 92°41.431′ | Yes | No | 51 | - | 24 | Pasture/Agriculture |
| Mexico | 2018 | Las Brisas de Hueyate | 15°1.422′ | 92°43.166′ | Yes | No | 113 | 114 | 0 | Mangrove |
| Mexico | 2018 | Manguito | 15°45.18′ | 93°30.99′ | No | No | 0 | - | 0 | Mangrove |
| Mexico | 2018 | Rancho el Piñon | 16°0.667′ | 93°40.2′ | No | No | 2 | 4 | 0 | Built-up rural |
| Mexico | 2018 | Ponte Duro | 15°48.485′ | 93°35.728′ | No | No | 3 | 4 | 3 | Built-up rural |
| Mexico | 2018 | Ponte Duro Mangroves | 15°49.063′ | 93°36.816′ | Yes | No | 16 | - | 8 | Mangrove |
| Mexico | 2018 | Puerto Arista | 15°56.98′ | 93°48.871′ | No | No | 0 | - | 0 | Built-up rural |
| Mexico | 2018 | Roberto Barrios | 15°20.435′ | 93°1.412′ | Yes | Yes | 23 | - | 4 | Pasture/Agriculture |
| Mexico | 2018 | Salto de Agua | 15°33.326′ | 93°11.633′ | Yes | Yes | 12 | - | 33 | Pasture/Agriculture |
| Mexico | 2019 | Aztlan Town Roost | 15°1.003′ | 92°42.278′ | Yes | Yes | 33 | 74 | 19 | Built-up rural |
| Mexico | 2019 | Las Palmas | 14°59.943′ | 92°41.455′ | Yes | No | 39 | 40 | 0 | Built-up rural |
| Mexico | 2019 | Huixtla | 15°3.233′ | 92°32.991′ | No | No | 0 | - | 41 | Built-up rural |
| Mexico | 2019 | Hidalgo | 15°5.872′ | 92°38.266′ | No | No | 0 | - | 0 | Built-up rural |
| Mexico | 2019 | Tapachula | 14°55.965′ | 92°13.33′ | No | No | 0 | - | 0 | Built-up rural |
| Guatemala | 2019 | Tilapa | 14°30.18′ | 92°10.753′ | No | No | 0 | - | 8 | Built-up rural |
| Guatemala | 2019 | San Martín Zapotitlan | 14°37.303′ | 91°32.358′ | No | No | 0 | - | 3 | Built-up rural |
| Guatemala | 2019 | Finca Patrocinio | 14°40.221′ | 91°36.538′ | No | No | 5 | 10 | 2 | Tropical dry |
| Guatemala | 2019 | Los Tarrales | 14°31.328′ | 91°8.34′ | No | No | 3 | 8 | 9 | Tropical dry |
| Guatemala | 2019 | Pineapple Plantation | 14°23.458′ | 91°5.732′ | Yes | No | 8 | 12 | 43 | Pasture/Agriculture |
| Honduras | 2019 | Sandy Bay | 16°19.133′ | 86°34.716′ | No | No | 22 | - | 55 | Built-up rural |
| Honduras | 2019 | Guava Grove | 16°18.985′ | 86°34.701′ | Yes | Yes | 20 | 24 | 4 | Pasture/Agriculture |
| Honduras | 2019 | Mud Hole | 16°20.81′ | 86°31.843′ | No | No | 0 | - | 0 | Built-up rural |
| Honduras | 2019 | Parrot Tree | 16°21.866′ | 86°24.851′ | No | No | 0 | - | 0 | Built-up rural |
| Honduras | 2019 | Los Fuertes | 16°20.913′ | 86°28.481′ | No | No | 0 | - | 0 | Built-up rural |
| Honduras | 2019 | Undisclosed | -- | -- | Yes | No | 248 | 266 | 0 | Tropical dry |
| Honduras | 2019 | Port Royal National Park | 16°25.083′ | 86°17.8′ | Yes | No | 10 | - | 10 | Tropical pine forest |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupin, M.K.; Dahlin, C.R.; Wright, T.F. Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata). Diversity 2020, 12, 377. https://doi.org/10.3390/d12100377
Dupin MK, Dahlin CR, Wright TF. Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata). Diversity. 2020; 12(10):377. https://doi.org/10.3390/d12100377
Chicago/Turabian StyleDupin, Molly K., Christine R. Dahlin, and Timothy F. Wright. 2020. "Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata)" Diversity 12, no. 10: 377. https://doi.org/10.3390/d12100377
APA StyleDupin, M. K., Dahlin, C. R., & Wright, T. F. (2020). Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata). Diversity, 12(10), 377. https://doi.org/10.3390/d12100377