First Survey of Heterobranch Sea Slugs (Mollusca, Gastropoda) from the Island Sangihe, North Sulawesi, Indonesia
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
References
- Suharsono. Jenis-Jenis Karang di Indonesia (Corals of Indonesia). In COREMAP Program; Indonesian Institute of Sciences (LIPI) Pusat Penelitian Oseanografi: Jakarta, Indonesia, 2008; p. 382. [Google Scholar]
- Wilkinson, C. Status of Coral Reefs of the World; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, Australia, 2008; p. 152. [Google Scholar]
- Suharsono. Condition of coral reef resources in Indonesia. J. Pesisir Lautan 1998, 1, 44–52. [Google Scholar]
- Utama, R.S.; Hadi, T.A. Recent coral reef conditions in Weh Island, Aceh Province, Indonesia. Ocean. Life 2018, 2, 47–53. [Google Scholar]
- Bintoro, G. Building resilience for communities in the face of damaged coastal ecosystems: A case study in Gerokgak Village, Buleleng Regency, Bali, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2018, 239, 012042. [Google Scholar]
- Perkembangan Pariwisata Sulawesi Utara Bulan Februari 2018. Available online: Sulut.bps.go.id/pressrelease/2018/04/02/393/perkembangan-pariwisata-sulawesi-utara-bulan-februari-2018.html (accessed on 1 May 2019).
- Setiawan, F.; Kusen, J.A.; Kaligis, G.J.F. Community change of coral reef fishes in Bunaken National Park, North Sulawesi, Indonesia (Perubahan struktur ikan karang di Taman Nasional Bunaken, Sulawesi Utara). Aquat. Sci. Manag. 2013, 1, 117–123. [Google Scholar]
- Towoliu, R. Kondisi terumbu karang pada beberapa pusat penyelamatan di Bunaken, Sulawesi Utara (Coral reef condition in several dive points around coral Bunaken island, North Sulawesi). Aquat. Sci. Manag. 2014, 2, 44–48. [Google Scholar]
- Indonesia Tourism. Available online: http://www.indonesia-tourism.com/northsulawesi/sangihe_archipelago.html (accessed on 3 May 2019).
- East Asia Minerals Corporation. Available online: https://eastasiaminerals.com/projects-indonesia/sangihe-gold-project/ (accessed on 10 May 2019).
- Kabupaten Kepulauan Sangihe Dalam Angka 2017. Available online: https://sangihekab.bps.go.id/publication/2017/08/13/18b493674a849a55b6a0b5ee/kabupaten-kepulauan-sangihe-dalam-angka-2017.html (accessed on 13 August 2017).
- Burghardt, I.; Carvalho, R.; Eheberg, D.; Gerung, G.; Kaligis, F.; Mamangkey, G.; Schroedl, M.; Schwabe, E.; Vonnemann, V.; Waegele, H. Molluscan diversity at Bunaken National Park, Sulawesi. J. Zool. Soc. Wallacea 2006, 2, 29–43. [Google Scholar]
- Kaligis, F.; Eisenbarth, J.H.; Schillo, D.; Dialao, J.; Schäberle, T.F.; Böhringer, N.; Bara, R.; Reumschüssel, S.; König, G.M.; Wägele, H. Second survey of heterobranch sea slugs (Mollusca, Gastropoda, Heterobranchia) from Bunaken national Park, North Sulawesi, Indonesia—How much do we know after 12 years. Mar. Biodiver. Rec. 2018, 11, 2. [Google Scholar] [CrossRef]
- Eisenbarth, J.H.; Undap, N.; Papu, A.; Schillo, D.; Dialao, J.; Reumschüssel, S.; Kaligis, F.; Bara, R.; Schäberle, T.F.; König, G.M.; et al. Marine Heterobranchia (Gastropoda, Mollusca) in Bunaken National Park, North Sulawesi, Indonesia—A follow-up diversity study. Diversity 2018, 10, 127. [Google Scholar] [CrossRef]
- Ompi, M.; Lumoindong, F.; Undap, N.; Papu, A.; Wägele, H. Monitoring marine Heterobranchia in Lembeh Strait, North Sulawesi (Indonesia), in a changing environment. AACL Bioflux 2019, 12, 664–667. [Google Scholar]
- Nimbs, M.J.; Larkin, M.; Davis, T.R.; Harasti, D.; Willan, R.C.; Smith, S.D.A. Southern range extensions for twelve heterobranch sea slugs (Gastropoda: Heterobranchia) on the eastern coast of Australia. Mar. Biodiv. Rec. 2016, 9, 27. [Google Scholar] [CrossRef]
- Nimbs, M.J.; Smith, D.A. Beyond Capricornia: Tropical sea slugs (Gastropoda, Heterobranchia) extend their distributions into the Tasman Sea. Diversity 2018, 10, 99. [Google Scholar] [CrossRef]
- Debelius, H.; Kuiter, R.H. Nacktschnecken der Weltmeere; IKAN Unterwasserarchiv: Frankfurt, Germany, 2007; p. 359. [Google Scholar]
- Gosliner, T.M.; Behrens, D.W.; Valdès, A. Indo-Pacific Nudibranchs and Sea Slugs: A Field Guide to the World’s Most Diverse Fauna; Sea Challengers: San Francisco, CA, USA, 2008; p. 425. [Google Scholar]
- Gosliner, T.M.; Valdès, A.; Behrens, D.W. Nudibranch and Sea Slug Identification Indo-Pacific; New World Publications: Jacksonville, FL, USA, 2015; p. 408. [Google Scholar]
- Rudman, W.B. The Chromodorididae (Opisthobranchia, Mollusca) of the Indo-West Pacific: Chromodoris quadricolor, C. lineolata and Hypselodoris nigrolineata color groups. Zool. J. Linn. Soc. 1982, 76, 183–241. [Google Scholar] [CrossRef]
- Rudman, W.B. The Chromodorididae (Opisthobranchia, Mollusca) of the Indo-West Pacific: A review of the genera. Zool. J. Linn. Soc. 1984, 81. [Google Scholar] [CrossRef]
- Rudman, W.B. The Chromodorididae (Opisthobranchia: Mollusca) of the Indo-West Pacific: The genus Glossodoris Ehrenberg (Casella, H. & A. Adams). Zool. J. Linn. Soc. 1986, 86, 101–184. [Google Scholar]
- Rudman, W.B. The Chromodorididae (Opisthobranchia, Mollusca) of the Indo-West Pacific: Chromodoris epicuria, C. aureopurpurea, C. annulata, C. coi and Risbecia tryoni color groups. Zool. J. Linn. Soc. 1987, 90, 305–407. [Google Scholar] [CrossRef]
- Yonow, N. Opisthobranchs from the Maldive Islands, including descriptions of seven new species (Mollusca: Gastropoda). Rev. Franç. Aquar. Herpet 1994, 20, 97–130. [Google Scholar]
- Yonow, N. Systematic Revision of the Family Phyllidiidae in the Indian Ocean Province: Part 1 (Opisthobranchia: Nudibranchia: Doridoidea). J. Conch. 1996, 35, 483–516. [Google Scholar]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 11. Doridacea of the families Chromodorididae and Hexabranchidae (Mollusca, Gastropoda, Opisthobranchia, NudibranchiaI). Zool. Meded. 2001, 75, 1–50. [Google Scholar]
- Yonow, N. Sea Slugs of the Red Sea; Pensoft Publishers: Sofia, Bulgaria, 2008; p. 304. [Google Scholar]
- Yonow, N. Results of the Rumphius Biohistorical Expedition to Ambon (1990). Part 15. The suborder Doridina (Mollusca, Gastropoda, Opisthobranchia, Nudibranchia). Zool. Meded. 2011, 85, 905–956. [Google Scholar]
- Yonow, N. Opisthobranchs from the western Indian Ocean, with descriptions of two new species and ten new records (Mollusca, Gastropoda). ZooKeys 2012, 197, 1–129. [Google Scholar] [CrossRef]
- The Sea Slug Forum. Available online: www.seaslugforum.net (accessed on 05 June 2019).
- WoRMS Editorial Board. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 10 June 2019).
- Diepenbroek, M.; Glöckner, F.; Grobe, P.; Güntsch, A.; Huber, R.; König-Ries, B.; Kostadinov, I.; Nieschulze, J.; Seeger, B.; Tolksdorf, R.; et al. Towards an Integrated Biodiversity and Ecological Research Data Management and Archiving Platform: The German Federation for the Curation of Biological Data (GFBio). Informatik 2014, 232, 1711–1724. [Google Scholar]
- Astrin, J.J.; Stüben, P.E. Phylogeny in cryptic weevils: Molecules, morphology and genera of western Palaearctic Cryptorhynchinae (Coleoptera: Curculionidae); Invertebrate Systematics; CSIRO Publishing: Clayton, Australia, 2008; pp. 503–522. [Google Scholar]
- Palumbi, S.R.; Martin, A.; Romano, S.; MacMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR; Version 2.0; Department of Zoology and Kewalo Marine Laboratory, University of Hawaii: Honolulu, HI, USA, 2002; p. 45. [Google Scholar]
- GBIF.org. Available online: https://doi.org/10.15468/dl.mhkexk (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.a8jpj7 (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.3vlodn (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.u1dqtv (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.2jdtpp (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.8sm78 (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.lycuc9 (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.flgtfy (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.2xa8go (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.3fmoxu (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.xplg0z (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.uzsfrc (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.hhqftx (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.4lqgzh (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.nhnbso (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.y94qqg (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.108oy5 (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.rgidut (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.unlgk3 (accessed on 19 August 2019).
- GBIF.org. Available online: https://doi.org/10.15468/dl.mimhpp (accessed on 19 August 2019).
- Meyers-Muñoz, M.A.; van der Velde, G.; van der Meij, S.E.T.; Stoffels, B.E.M.W.; van Alen, T.; Tuti, Y.; Hoeksema, B.W. The phylogenetic position of a new species of Plakobranchus from West Papua, Indonesia (Mollusca, Opisthobranchia, Sacoglossa). ZooKeys 2016, 594, 73–98. [Google Scholar]
- Yonow, N.; Jensen, K.R. Result of the Rumphius Biohistorical Expedition to Ambon (1990). Part 17. The Cephalaspidea, Anaspidea, Pleurobranchida, and Sacoglossa (Mollusca: Gastropoda: Heterobranchia). Arch. Molluskenkd. 2018, 147, 1–48. [Google Scholar] [CrossRef]
- Gosliner, T.M.; Behrens, D.W. Five new species of Chromodoris (Mollusca: Nudibranchia; Chromodorididae) from the tropical Indo-West Pacific. Proc. Calif. Acad. Sci. 1998, 50, 139–165. [Google Scholar]
- Cheney, K.L.; White, A.; Mudianta, I.W.; Winters, A.E.; Quezada, M.; Capon, R.J.; Mollo, E.; Garson, M.J. Choose your weaponry: Selective storage of a single toxic compound, Latrunculin A, by closely related nudibranch molluscs. PLoS ONE 2016, 11, e0145134. [Google Scholar] [CrossRef] [PubMed]
- Padula, V.; Bahia, J.; Stöger, I.; Camacho-Garcia, Y.; Malaquias, M.A.E.; Cervera, J.L.; Schrödl, M. A test of color-base taxonomy in nudibranchs: Molecular Phylogeny and species delimitation of the Felimida clenchi (Mollusca: Chromodorididae) species complex. Mol. Phys. Evol. 2016, 103, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Layton, K.K.S.; Gosliner, T.M.; Wilson, N.G. Flexible color patterns obscure identification and mimicry in Indo–Pacific Chromodoris nudibranchs (Gastropoda: Chromodorididae). Mol. Phyl. Evol. 2018, 124, 27–36. [Google Scholar] [CrossRef]
- Johnson, R.F.; Gosliner, T.M. Traditional taxonomic groupings mask evolutionary history: A molecular phylogeny and new classification of the chromodorid nudibranchs. PLoS ONE 2012, 7, e33479. [Google Scholar] [CrossRef]
- Matsuda, S.B.; Gosliner, T.M. Glossing over cryptic species: Descriptions of four new species of Glossodoris and three new species of Doripsimatica (Nudibranchia: Chromodorididae). Zootaxa 2018, 4444, 501–529. [Google Scholar] [CrossRef]
- Yonow, N. Red Sea Opisthobranchia 5: New species and new records of chromodorids from the Red Sea (Heterobranchia, Nudibranchia, Chromodorididae). ZooKeys 2018, 770, 9–42. [Google Scholar] [CrossRef]
- Bergh, L.S.R. Malacologische Untersuchungen. In Reisen imArchipel der Philippinen. Zweiter Theil; Semper, C., Ed.; Wissenschaftliche Resultate: Berlin, Germany, 1888; pp. 815–872. [Google Scholar]
- Tibiriçá, Y.; Pola, M.; Cervera, J.L. Astonishing diversity revealed: An annotated and illustrated inventory of Nudipleura (Gastropoda: Heterobranchia) from Mozambique. Zootaxa 2017, 1, 1–133. [Google Scholar] [CrossRef]
- Brunckhorst, D.J. The systematics and phylogeny of Phyllidiid Nudibranch (Doridoidea). Rec. Austr. Mus. Suppl. 1993, 16, 1–107. [Google Scholar] [CrossRef]
- Stoffels, B.E.M.W.; van der Meij, S.E.T.; Hoeksema, B.W.; van Alphen, J.; van Alen, T.; Meyers-Munoz, M.A.; de Voogd, N.J.; Tuti, Y.; van der Velde, G. Phylogenetic relationships within the Phyllidiidae (Opisthobranchia, Nudibranchia). ZooKeys 2016, 605, 1–35. [Google Scholar]
- Rudman, W.B. Comment on Phyllidia madangensis from New Caledonia by Jean-François Hervé. Available online: http://www.seaslugforum.net (accessed on 16 June 2019).
- Brunckhorst, D.J. Redescription of Phyllidia coelestis Bergh, 1905 (Ophisthobranchia: Nudibranchia: Doridoidea). J. Malac. Soc. Aust. 1989, 10, 35–45. [Google Scholar]
- Carmona, L.; Malaquias, M.A.E.; Gosliner, T.M.; Pola, M.; Cervera, J.L. Amphi-Atlantic Geographic distributions and cryptic species in Sacoglossan sea slugs. J. Moll. Stud. 2011, 77, 401–412. [Google Scholar] [CrossRef]
- Ornelas-Gatdula, E.; Dupont, A.; Valdès, A. The tail tells the tale; taxonomy and biogeography of some Atlantic Chelidonura (Gastropoda; Cephalaspidea; Aglajidae) inferred from nuclear and mitochondrial gen data. Zool. J. Linn. Soc. 2011, 163, 1077–1095. [Google Scholar] [CrossRef]
- Pola, M.; Camacho-García, Y.E.; Gosliner, T.M. Molecular data illuminate cryptic nudibranch species: The evolution of the Scyllaeidae (Nudibranchia; Dendrotina) with a revision of Notobryon. Zool. J. Linn. Soc. 2012, 165, 311–336. [Google Scholar] [CrossRef]
- Pola, M.; Roldan, P.; Padilla, S. Molecular data on the genus Okenia (Nudibranchia; Goniodorididae) reveal a new cryptic species from New South Wales (Australia). J. Mar. Biol. Assoc. UK 2014, 94, 587–598. [Google Scholar] [CrossRef]
- Matsuda, S.B.; Gosliner, T.M. Molecular phylogeny of Glossodoris (Ehrenberg, 1831) nudibranchs and related genera reveals cryptic and pseudocryptic species complexes. Cladistics. 2017, 34, 41–56. [Google Scholar] [CrossRef]
- Epstein, H.E.; Hallas, J.M.; Johnson, R.F.; Lopez, A.; Gosliner, T.M. Reading between the lines: Revealing cryptic species diversity and color patterns in Hypselodoris nudibranchs (Mollusca: Heterobranchia: Chromodorididae). Zool. J. Linnean Soc. 2018, 20, 116–189. [Google Scholar] [CrossRef]
- Yonow, N.; Hayward, P.J. Opisthobranches de I’lle Maurice, avec la description de deux espèces nouvelles (Mollusca: Opithobranchia). Rev. Franç Aquar. Herpet 1991, 18, 1–30. [Google Scholar]
- Wägele, H. Potential key characters in Opisthobranchia (Gastropoda, Mollusca) enhancing adaptive radiation. Org. Divers. Evol. 2004, 4, 175–188. [Google Scholar]
- Goodheart, J.A.; Ellingson, R.A.; Vital, X.G.; Filho, H.C.; McCarthy, J.B.; Medrano, S.M.; Bhave, V.J.; Mendez, K.G.; Jimenez, L.M.; Lopez, G.; et al. Identification guide to the heterobranch sea slugs (Mollusca: Gastropoda) from Bocas del Toro, Panama. Mar. Biodiv. Rec. 2016, 9, 56. [Google Scholar] [CrossRef]
- Yonow, N. Results from the Rumphius Biohistorical Expedition to Ambon (1990). Part 16. Nudibranchia- Dendronotina, Arminina, Aeolidina, and Doridina (Mollusca, Gastropoda, Heterobranchia). Arch. Molluskenkd. 2017, 146, 135–172. [Google Scholar]
- Tonozuka, T. Opisthobranchs of Bali and Indonesia; Hankyu Communications Co. Ltd.: Tokyo, Japan, 2003. [Google Scholar]
- Martynov, A.V.; Korshunova, T.A. Opisthobranch molluscs of Vietnam (Gastropoda: Opisthobranchia). In Benthic Fauna of the Bay of Nhatrang, Southern Vietnam; Britayev, T.A., Pavlov, D.S., Eds.; KMK Scientific Press: Moscow, Russia, 2012; Volume 2, pp. 142–257. [Google Scholar]
- Gosliner, T.M. Biodiversity of tropical opisthobranch gastropod faunas. Proc. Int. Coral Reef Symp. Guam 1992, 2, 702–709. [Google Scholar]
- Huang, H.-D.; Tsai, Y.-C.; Chen, C.-K.; Hung, H.-T.; Chen, J.-C.; Lin, H.; Lee, K.-S. Diverse opisthobranchs and polyclad flatworms in Houwan, Kenting National Park, Southern Taiwan. Coll. Res. 2015, 28, 55–65. [Google Scholar]
- Orr, J. Hong Kong Nudibranchs; Urban Council: Hong Kong, China, 1981. [Google Scholar]
- Yonow, N.; Anderson, R.A.; Buttress, S.G. Opisthobranch Molluscs from the Chagos Archipelago, Central Indian Ocean. J. Nat. Hist. 2002, 36, 831–882. [Google Scholar] [CrossRef]
- Johnson, S.; Boucher, L.M. Notes on some Opisthobranchia (Mollusca: Gastropoda) from the Marshall Islands, including 57 new records. Pac. Sci. 1983, 37, 251–291. [Google Scholar]
- Wägele, H.; Burghardt, I.; Anthes, N.; Evertsen, J.; Klussmann-Kolb, A.; Brodie, G. Species diversity of opisthobranch molluscs on Lizard Island, Great Barrier Reef, Australia. Rec. West. Austr. Mus. 2006, 69, 33–59. [Google Scholar] [CrossRef]
- Wells, F.E.; Bryce, C.W. Sea Slugs and Their Relatives of Western Australia; Western Australian Museum: Perth, Australia, 1993; p. 184. [Google Scholar]
- Brodie, G.D.; Brodie, J.E. A checklist of the opisthobranchs molluscs of Fiji. J. Malacol. Soc. Austr. 1990, 11, 53–63. [Google Scholar] [CrossRef]
- Bouchet, P.; Lozouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Marshall, J.G.; Willan, R.C. Nudibranchs of Heron Island, Great Barrier Reef; Backhuys Publishers: Leiden, The Netherlands, 1999; p. 268. [Google Scholar]
- Apte, D. Opisthobranch fauna of Lakshadweep Islands, India, with 52 new records to Lakshadweep and 40 new records to India: Part 1. J. Bombay Nat. Hist. Soc. 2009, 106, 162–175. [Google Scholar]
- Hervé, J.-F. Guide des Nudibranches de Nouvelle-Calédonie et Autres Opisthobranches; Editions Catherine Ledru: Nouméa, France, 2010; p. 404. [Google Scholar]
- Nimbs, M.J.; Smith, D.A. An illustrated inventory of the sea slugs of New South Wales, Australia (Gastropoda: Heterobranchia). R. Soc. Vic. 2016, 128, 44–113. [Google Scholar] [CrossRef]
- Bertsch, H. Biogeography of northeast Pacific opisthobranchs: Comparative faunal province studies between point conception, California, USA, and Punta Aguja, Piura, Perú. Persp. Malacol. Mexicana 2010, 13, 219–259. [Google Scholar]
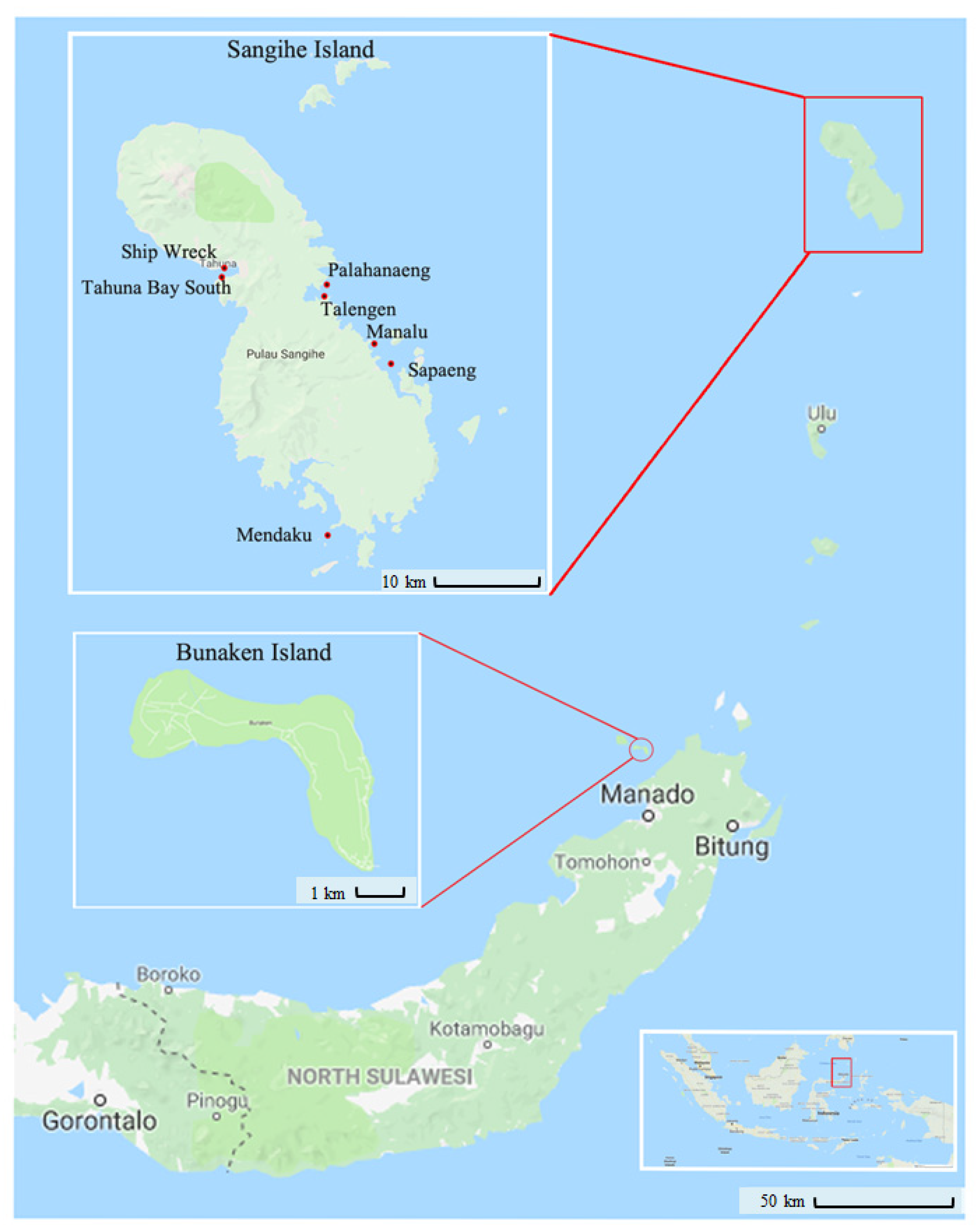



| Name | Abbreviation | Area and Geographic Data | Date of Collection |
|---|---|---|---|
| Ship Wreck | ShW | 3°36′28.00″ N 125°29′38.00″ E | 04.08.2016 |
| Tahuna Bay South | TBS | 3°35′59.40″ N 125°29′23.40″ E | 04.08.2016 |
| Mendaku | Men | 3°22′01.94″ N 125°34′26.67″ E | 03.08.2016 |
| Palahanaeng (village) | Pal | 3°35′18.92″ N 125°34′26.67″ E | 07.08.2016 |
| Talengen (village) | Tal | 3°34′49.92″ N 125°34′34.93″ E | 05.08.2016 |
| Manalu | Man | 3°32′08.87″ N 125°37′25.46″ E | 06.08.2016 |
| Sapaeng | Sap | 3°34′55.81″ N 125°34′49.04″ E | 06.08.2016 |
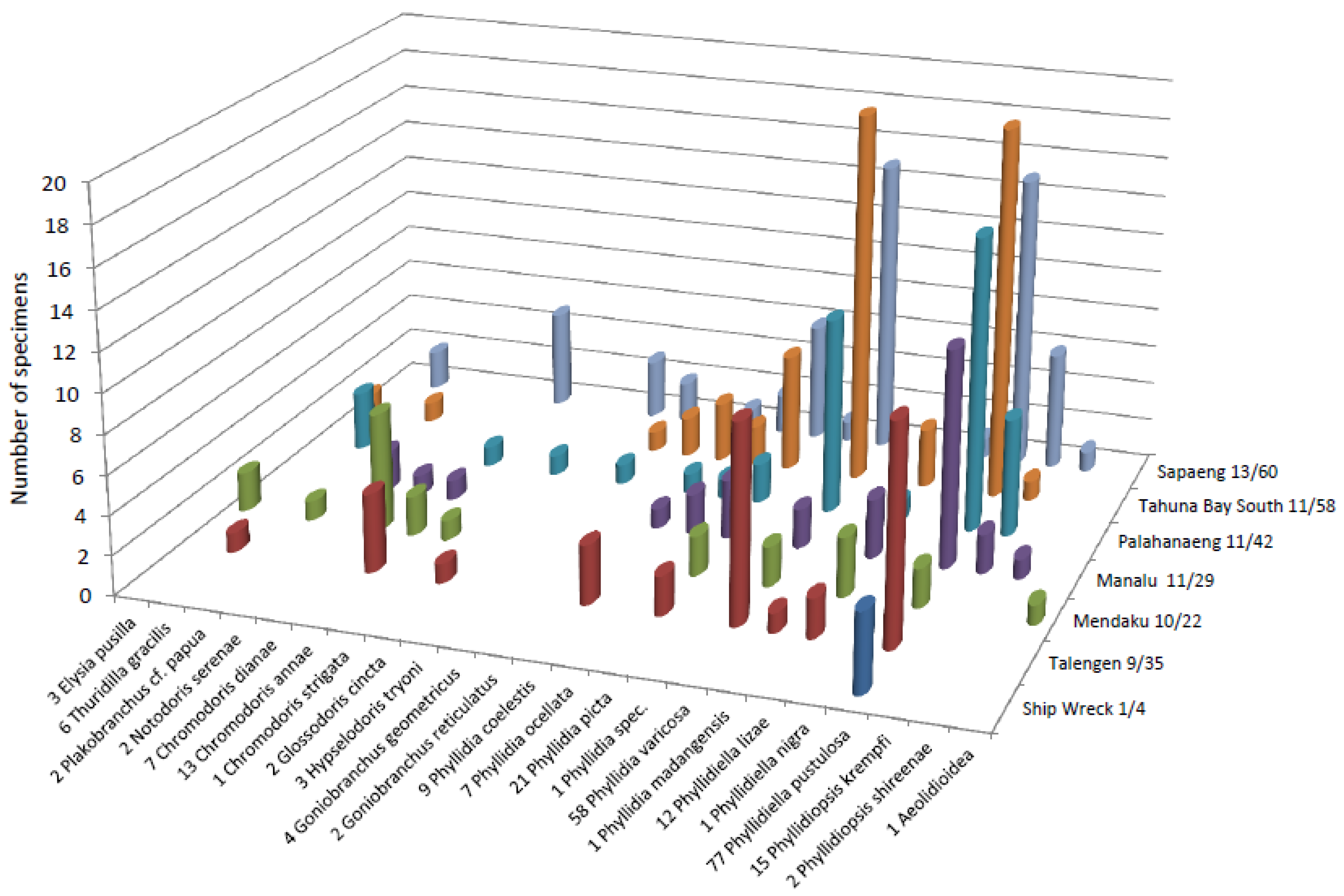
| Taxon | Species Name | Localities | Depths (m) | Number of Specimens | Size (mm) | Eisenbarth et al. [14] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBS | ShW | Man | Pal | Men | Sap | Tal | ||||||
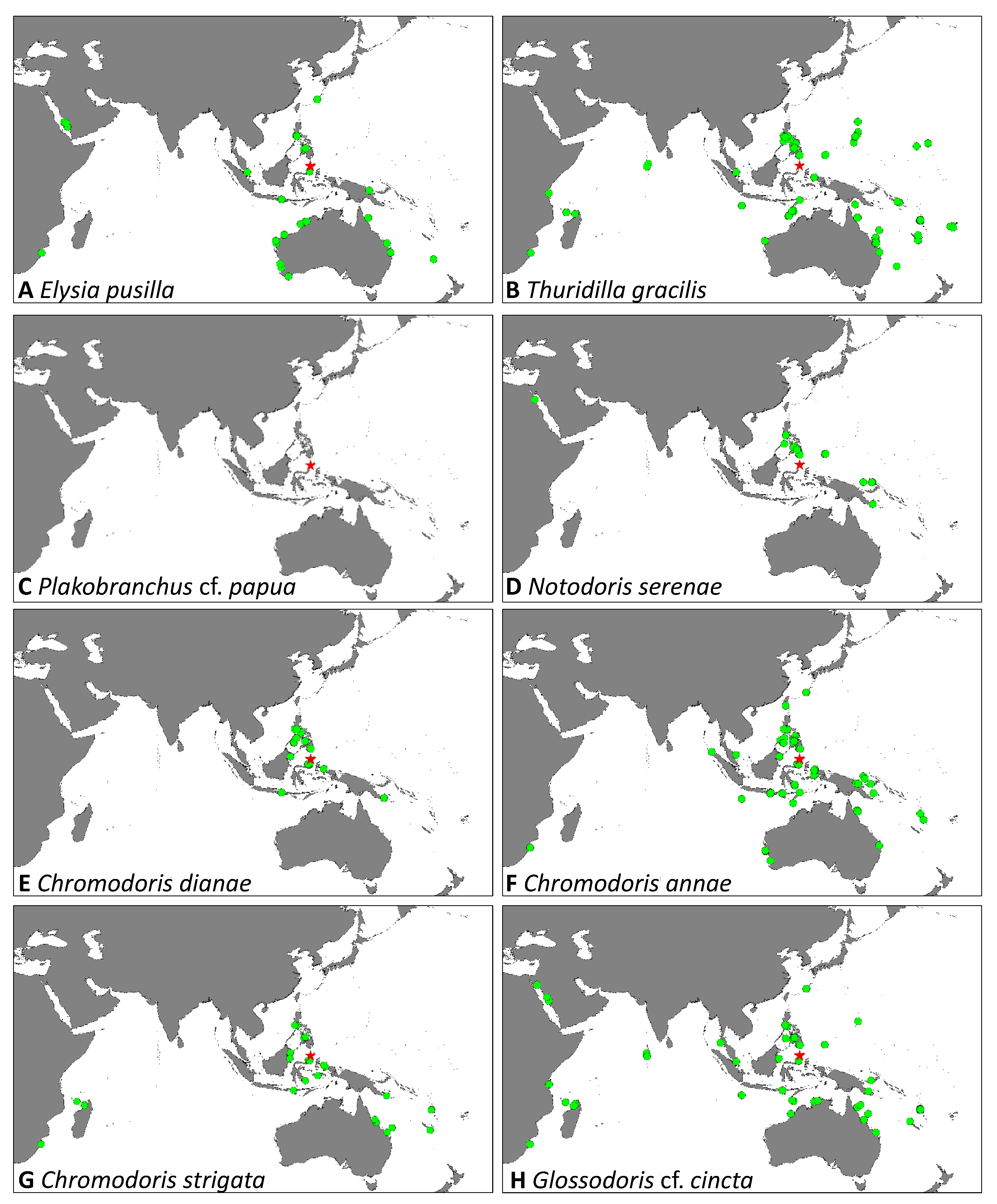
| Sacoglossa | Elysia pusilla (Bergh, 1871) | 1 | - | - | - | 2 | - | - | 2 | 3 | 2–6 | x |
| Thuridilla gracilis (Risbec, 1928) | - | - | - | 3 | - | 2 | 1 | 4–10 | 6 | 20–30 | x | |
| Plakobranchus cf. papua (Meyers-Muñoz & van der Velde, 2016) | 1 | - | - | - | 1 | - | - | 5–15 | 2 | 25,30 | - | |
| Anthobranchia | Notodoris serenae (Gosliner & Behrens, 1997) | - | - | 2 | - | - | - | - | 24–27 | 2 | 60,90 | x |
| Chromodoris dianae (Gosliner & Behrens, 1998) | - | - | 1 | - | 6 | - | - | 15–27 | 7 | 5–45 | x | |
| Chromodoris annae (Bergh, 1877) | - | - | 1 | 1 | 2 | 5 | 4 | 5–23 | 13 | 8–41 | x | |
| Chromodoris strigata (Rudman, 1982) | - | - | - | - | 1 | - | - | 15 | 1 | 10 | x | |
| Glossodoris cf. cincta (Bergh, 1888) | - | - | - | 1 | - | - | 1 | 8, 13 | 2 | 21, 48 | x | |
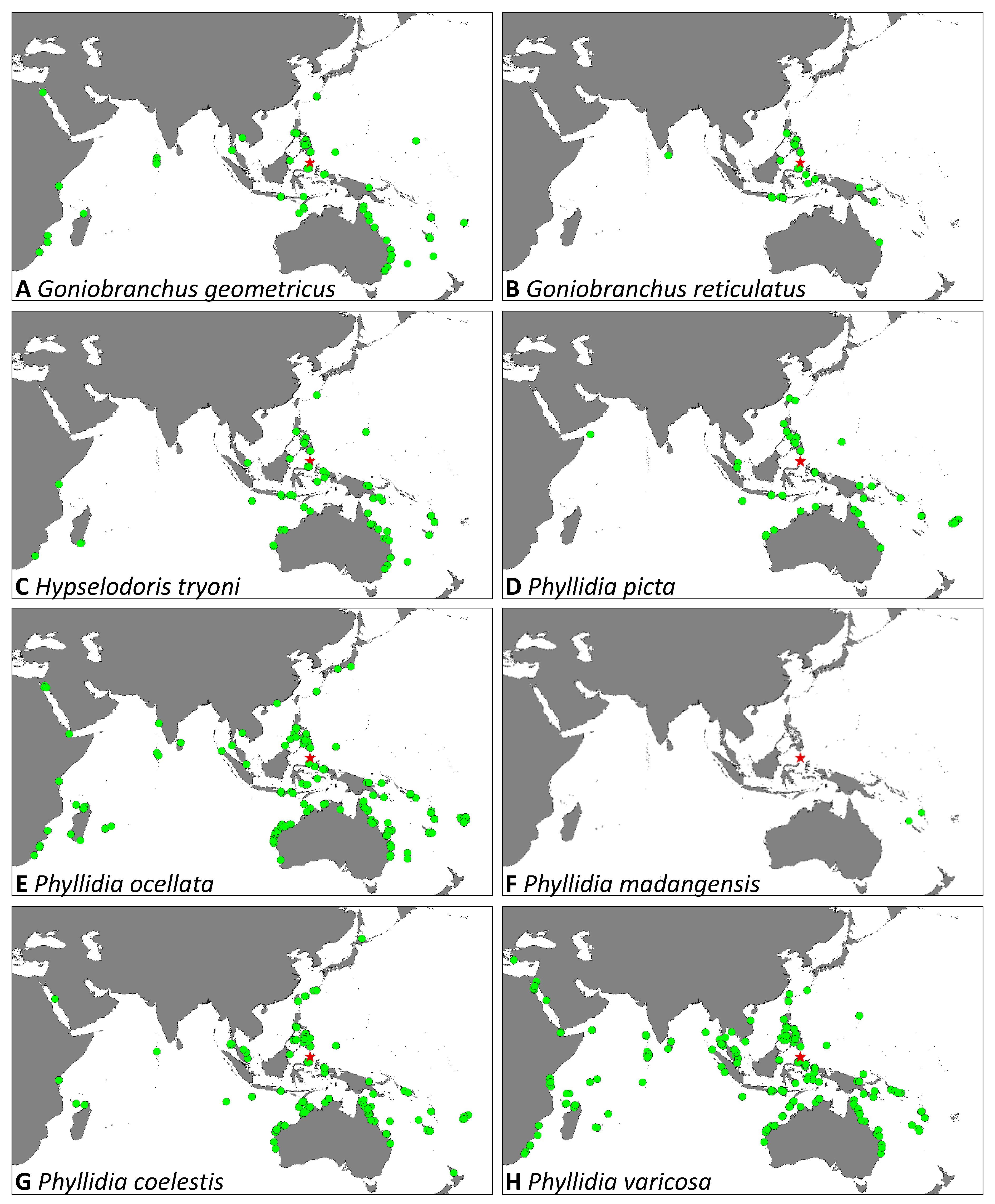
| Goniobranchus geometricus (Risbec, 1928) | 1 | - | - | 1 | - | 2 | - | 6–19 | 4 | 10–15 | x | |
| Goniobranchus reticulatus (Quoy & Gaimard, 1832) | 2 | - | - | - | - | - | - | 6, 9 | 2 | 25, 55 | x | |
| Hypselodoris tryoni (Garret, 1873) | - | - | - | - | - | 3 | - | 10, 16 | 3 | 25–60 | x | |
| Phyllidia ocellata (Cuvier, 1804) | 2 | - | 2 | 1 | - | 2 | - | 4–18 | 7 | 16–35 | x | |
| Phyllidia picta (Pruvot-Fol, 1957) | 6 | - | 3 | 2 | 2 | 6 | 2 | 1–15 | 21 | 13–30 | - | |
| Phyllidia spec. (Phsp3_16Sa-1) | - | - | - | - | - | 1 | - | 1 | 1 | 25 | - | |
| Phyllidia madangensis (Brunckhorst, 1993) | - | - | - | - | - | - | 1 | 8 | 1 | 28 | - | |
| Phyllidia coelestis (Bergh, 1905) | 3 | - | 1 | 1 | - | 1 | 3 | 3–12 | 9 | 7–32 | x | |
| Phyllidia varicose (Lamarck, 1801) | 19 | - | 2 | 10 | 2 | 15 | 10 | 3–15 | 58 | 7–87 | x | |
| Phyllidiella lizae (Brunckhorst, 1993) | 3 | - | 3 | 1 | 3 | - | 2 | 5–23 | 12 | 6–68 | - | |
| Phyllidiella pustulosa (Cuvier, 1804) | 19 | 4 | 11 | 15 | 2 | 15 | 11 | 1–23 | 77 | 12–47 | x | |
| Phyllidiella nigra (van Hasselt, 1824) | - | - | - | - | - | 1 | - | 8 | 1 | 29 | x | |
| Phyllidiopsis krempfi (Pruvot-Fol, 1957) | 1 | - | 2 | 6 | - | 6 | - | 6–28 | 15 | 14–50 | - | |
| Phyllidiopsis shireenae (Brunckhorst, 1990) | - | - | 1 | - | - | 1 | - | 8, 15 | 2 | 77, 81 | - | |
| Cladobranchia | Aeolidioidea (Flsp16Sa-1) | - | - | - | - | 1 | - | - | 2 | 1 | 1 | - |
| Family | Species Name | ID | GenBank Accession Numbers | |
|---|---|---|---|---|
| 16 S | CO1 | |||
| Chromodorididae (Bergh, 1891) | Chromodoris dianae (Gosliner & Behrens, 1998) | Chdi16Sa-1 | MN104702 | MN320502 |
| Chdi16Sa-2 | MN104703 | MN320503 | ||
| Chdi16Sa-3 | MN104704 | MN320504 | ||
| Chdi16Sa-4 | MN104705 | MN320505 | ||
| Chdi16Sa-5 | MN104706 | MN320506 | ||
| Chdi16Sa-6 | MN104707 | MN320507 | ||
| Chdi16Sa-7 | MN104708 | MN320508 | ||
| Chromodoris annae (Bergh, 1877) | Chan16Sa-1 | MN104690 | MN124751 | |
| Chan16Sa-2 | MN104691 | MN124752 | ||
| Chan16Sa-3 | MN104692 | MN124753 | ||
| Chan16Sa-4 | MN104693 | MN124754 | ||
| Chan16Sa-5 | MN104694 | MN124755 | ||
| Chan16Sa-6 | MN104695 | MN124756 | ||
| Chan16Sa-7 | MN104696 | MN124757 | ||
| Chan16Sa-8 | MN104698 | MN124758 | ||
| Chan16Sa-9 | MN104699 | MN124759 | ||
| Chan16Sa-10 | MN104700 | MN124760 | ||
| Chan16Sa-11 | MN104701 | MN124761 | ||
| Chan16Sa-12 | MN104702 | MN124762 | ||
| Chel16Sa-1 | MN104709 | MN124763 | ||
| Chromodoris strigata (Rudman, 1982) | Chst16Sa-1 | MN104710 | MN365022 | |
| Glossodoris cf. cincta (Bergh, 1888) | Glci16Sa-1 | MN104711 | MN339440 | |
| Glci16Sa-2 | MN104712 | MN339441 | ||
| Goniobranchus geometricus (Risbec, 1928) | Goge16S-1 | MN104715 | MN339442 | |
| Goge16S-2 | MN104716 | MN339443 | ||
| Goge16S-3 | MN104717 | MN339444 | ||
| Goge16S-4 | - | MN339445 | ||
| Goniobranchus reticulatus (Quoy & Gaimard, 1832) | Gore16Sa-1 | MN104719 | MN339446 | |
| Gore16Sa-2 | MN104720 | MN339447 | ||
| Hypselodoris tryoni (Garret, 1873) | Goca16S-1 | MN104713 | MN339448 | |
| Goca16S-2 | MN104714 | MN339450 | ||
| Goku16Sa1 | MN104718 | MN339449 | ||
| Phyllidiidae (Rafinesque, 1814) | Phyllidia picta (Pruvot-Fol, 1957) | Phpic16Sa-1 | MN217674 | MN248545 |
| Phpic16Sa-5 | MN217680 | MN248543 | ||
| Phpic16Sa-6 | MN217675 | MN248546 | ||
| Phpic16Sa-8 | MN217671 | MN248540 | ||
| Phpic16Sa-9 | MN217669 | MN248539 | ||
| Phpic16Sa-10 | MN217672 | MN248542 | ||
| Phpic16Sa-11 | MN217679 | MN248549 | ||
| Phpic16Sa-12 | MN217678 | MN248547 | ||
| Phpic16Sa-13 | MN217676 | MN248548 | ||
| Phpic16Sa-14 | MN217681 | MN248544 | ||
| Phsp616Sa-3 | MN217677 | MN248550 | ||
| Phspec116Sa-2 | MN217670 | MN248541 | ||
| Phyllidia spec. | Phsp316Sa-1 | MN217673 | MN265389 | |
| Phyllidia ocellata (Cuvier, 1804) | Phoc16S-1 | MN173896 | MN173896 | |
| Phoc16S-2 | MN173895 | MN173895 | ||
| Phoc16S-4 | MN173894 | MN173894 | ||
| Phoc16S-5 | MN173893 | MN173893 | ||
| Phoc16S-6 | - | MN173892 | ||
| Phoc16S-7 | MN173891 | MN173891 | ||
| Phyllidia coelestis (Bergh, 1905) | Phco16Sa-1 | MN172238 | MN234119 | |
| Phco16Sa-2 | MN172237 | MN234113 | ||
| Phco16Sa-3 | MN172236 | MN234115 | ||
| Phco16Sa-4 | MN172235 | MN234118 | ||
| Phco16Sa-5 | MN172234 | MN234112 | ||
| Phco16Sa-7 | MN172233 | MN234116 | ||
| Phco16Sa-9 | MN172232 | MN234114 | ||
| Phco16Sa-10 | MN172231 | MN234112 | ||
| Phyllidia varicosa (Lamarck, 1801) | Phva16Sa-2 | MN243776 | - | |
| Phva16Sa-3 | MN243779 | - | ||
| Phva16Sa-4 | MN243778 | MN248554 | ||
| Phva16Sa-5 | MN243774 | - | ||
| Phva16Sa-7 | MN243747 | - | ||
| Phva16Sa-8 | MN243735 | - | ||
| Phva16Sa-9 | MN243783 | MN248572 | ||
| Phva16Sa-10 | MN243750 | - | ||
| Phva16Sa-11 | MN243761 | - | ||
| Phva16Sa-12 | MN243781 | - | ||
| Phva16Sa-13 | MN243760 | MN248571 | ||
| Phva16Sa-15 | MN243782 | MN248555 | ||
| Phva16Sa-16 | MN243775 | - | ||
| Phva16Sa-17 | MN243759 | - | ||
| Phva16Sa-18 | MN243780 | - | ||
| Phva16Sa-20 | MN243758 | MN248556 | ||
| Phva16Sa-21 | MN243734 | MN248563 | ||
| Phva16Sa-22 | MN243777 | - | ||
| Phva16Sa-23 | MN243773 | - | ||
| Phva16Sa-24 | MN243757 | MN248568 | ||
| Phva16Sa-25 | MN243746 | - | ||
| Phva16Sa-26 | MN243733 | - | ||
| Phva16Sa-27 | MN243771 | MN248573 | ||
| Phva16Sa-28 | MN243748 | - | ||
| Phva16Sa-29 | MN243745 | - | ||
| Phva16Sa-30 | MN243740 | - | ||
| Phva16Sa-31 | MN243770 | MN248567 | ||
| Phva16Sa-32 | MN243768 | - | ||
| Phva16Sa-33 | MN243767 | MN248574 | ||
| Phva16Sa-34 | MN243756 | - | ||
| Phva16Sa-36 | MN243772 | - | ||
| Phva16Sa-37 | MN243755 | MN248569 | ||
| Phva16Sa-38 | MN243763 | - | ||
| Phva16Sa-39 | MN243744 | - | ||
| Phva16Sa-40 | MN243754 | MN248557 | ||
| Phva16Sa-41 | MN243739 | - | ||
| Phva16Sa-42 | MN243749 | MN248562 | ||
| Phva16Sa-43 | MN243764 | MN248565 | ||
| Phva16Sa-44 | MN243766 | MN248561 | ||
| Phva16Sa-45 | MN243741 | MN248564 | ||
| Phva16Sa-46 | MN243738 | - | ||
| Phva16Sa-47 | MN243737 | MN248558 | ||
| Phva16Sa-48 | MN243753 | - | ||
| Phva16Sa-49 | MN243743 | - | ||
| Phva16Sa-50 | MN243742 | MN248559 | ||
| Phva16Sa-52 | MN243765 | MN248560 | ||
| Phva16Sa-53 | MN243762 | MN248570 | ||
| Phva16Sa-54 | MN243752 | - | ||
| Phva16Sa-55 | MN243751 | - | ||
| Phva16Sa-56 | MN243769 | MN248566 | ||
| Phva16Sa-57 | MN243736 | - | ||
| Phva16Sa-58 | MN243732 | - | ||
| Phyllidiella lizae (Brunckhorst, 1993) | Phli16Sa-1 | MN243971 | MN248575 | |
| Phli16Sa-2 | MN243973 | MN248577 | ||
| Phli16Sa-5 | MN243972 | MN248576 | ||
| Phli16Sa-6 | MN243974 | MN248578 | ||
| Phyllidiella pustulosa (Cuvier, 1804) | Phpu16Sa-1 | MN243977 | MN248624 | |
| Phpu16Sa-2 | MN244015 | MN248636 | ||
| Phpu16Sa-3 | MN243991 | MN248601 | ||
| Phpu16Sa-4 | MN243992 | MN248606 | ||
| Phpu16Sa-5 | MN243996 | MN248608 | ||
| Phpu16Sa-6 | MN244006 | MN248602 | ||
| Phpu16Sa-7 | MN244007 | MN248594 | ||
| Phpu16Sa-8 | MN243999 | - | ||
| Phpu16Sa-9 | MN243980 | MN248627 | ||
| Phpu16Sa-13 | MN243969 | MN248581 | ||
| Phpu16Sa-14 | - | MN248580 | ||
| Phpu16Sa-15 | MN243960 | MN248585 | ||
| Phpu16Sa-18 | MN243983 | MN248632 | ||
| Phpu16Sa-20 | - | MN248590 | ||
| Phpu16Sa-23 | MN243962 | MN248586 | ||
| Phpu16Sa-24 | MN243978 | MN248625 | ||
| Phpu16Sa-25 | MN244008 | MN248595 | ||
| Phpu16Sa-26 | MN243979 | MN248626 | ||
| Phpu16Sa-27 | MN244009 | MN248596 | ||
| Phpu16Sa-28 | MN243970 | MN248591 | ||
| Phpu16Sa-29 | MN243955 | MN248639 | ||
| Phpu16Sa-30 | MN244000 | MN248620 | ||
| Phpu16Sa-31 | MN243963 | MN248587 | ||
| Phpu16Sa-33 | MN243985 | MN248614 | ||
| Phpu16Sa-34 | MN244017 | MN248637 | ||
| Phpu16Sa-35 | MN243957 | MN248640 | ||
| Phpu16Sa-36 | MN244011 | MN248597 | ||
| Phpu16Sa-38 | MN243997 | MN248609 | ||
| Phpu16Sa-39 | MN244010 | MN248598 | ||
| Phpu16Sa-40 | MN243981 | MN248628 | ||
| Phpu16Sa-46 | MN244001 | MN248620 | ||
| Phpu16Sa-48 | MN243975 | MN248590 | ||
| Phpu16Sa-50 | MN243958 | MN248641 | ||
| Phpu16Sa-52 | MN244002 | MN248621 | ||
| Phpu16Sa-53 | MN243968 | MN248584 | ||
| Phpu16Sa-55 | MN244081 | - | ||
| Phpu16Sa-56 | MN243994 | MN248605 | ||
| Phpu16Sa-60 | MN244014 | MN248634 | ||
| Phpu16Sa-61 | MN244006 | MN248613 | ||
| Phpu16Sa-62 | MN243995 | MN248607 | ||
| Phpu16Sa-68 | - | MN248610 | ||
| Phpu16Sa-69 | MN244016 | MN248635 | ||
| Phpu16Sa-70 | MN244018 | MN248638 | ||
| Phpu16Sa-71 | MN243986 | MN248616 | ||
| Phpu16Sa-73 | MN243998 | - | ||
| Phpu16Sa-74 | MN243956 | MN248642 | ||
| Phpu16Sa-75 | MN243987 | MN248617 | ||
| Phpu16Sa-76 | MN243989 | MN248615 | ||
| Phpu16Sa-77 | MN243993 | MN248604 | ||
| Phpu16Sa-79 | MN243988 | MN248619 | ||
| Phpu16Sa-80 | MN244019 | MN248600 | ||
| Phpu16Sa-84 | MN244012 | MN248599 | ||
| Phpu16Sa-85 | MN243990 | MN248618 | ||
| Phpu16Sa-86 | MN244003 | MN248611 | ||
| Phpu16Sa-87 | MN243982 | MN248629 | ||
| Phpu16Sa-90 | - | MN248630 | ||
| Phpu16Sa-91 | MN243984 | MN248631 | ||
| Phpu16Sa-92 | MN243967 | MN248592 | ||
| Phpu16Sa-94 | - | MN248603 | ||
| Phpu16Sa-95 | MN244004 | MN248612 | ||
| Phli16Sa-4 | MN243976 | MN248623 | ||
| Phli16Sa-7 | MN244013 | MN248633 | ||
| Phyllidiella nigra (van Hasselt, 1824) | Phpu16Sa-64 | - | - | |
| Phyllidiopsis krempfi (Pruvot-Fol, 1993) | Phfi16Sa-1 | MN244067 | MN248643 | |
| Phfi16Sa-2 | MN244068 | MN248644 | ||
| Phpu16Sa-19 | - | MN248652 | ||
| Phpu16Sa-47 | MN244076 | MN248654 | ||
| Phpu16Sa-54 | MN244077 | MN248653 | ||
| Phpu16Sa-57 | MN244071 | MN248651 | ||
| Phpu16Sa-58 | MN244074 | MN248650 | ||
| Phpu16Sa-65 | MN244072 | MN248649 | ||
| Phpu16Sa-66 | MN244073 | MN248647 | ||
| Phpu16Sa-67 | MN244069 | MN248645 | ||
| Phpu16Sa-72 | MN244070 | MN248646 | ||
| Phpu16Sa-82 | - | MN248658 | ||
| Phpu16Sa-83 | MN244080 | MN248657 | ||
| Phpu16Sa-88 | MN244075 | MN248646 | ||
| Phpu16Sa-93 | MN244078 | MN248655 | ||
| Phyllidiopsis shireenae (Brunckhorst, 1990) | Phsh16Sa-2 | MN244082 | MN248659 | |
| Acteonoidea | Cephalaspidea + Runcinacea | Anaspidea | Sacoglossa | Umbraculida | Pleurobranchomorpha | Anthobranchia | Cladobranchia | Total Species Number | References | |
|---|---|---|---|---|---|---|---|---|---|---|
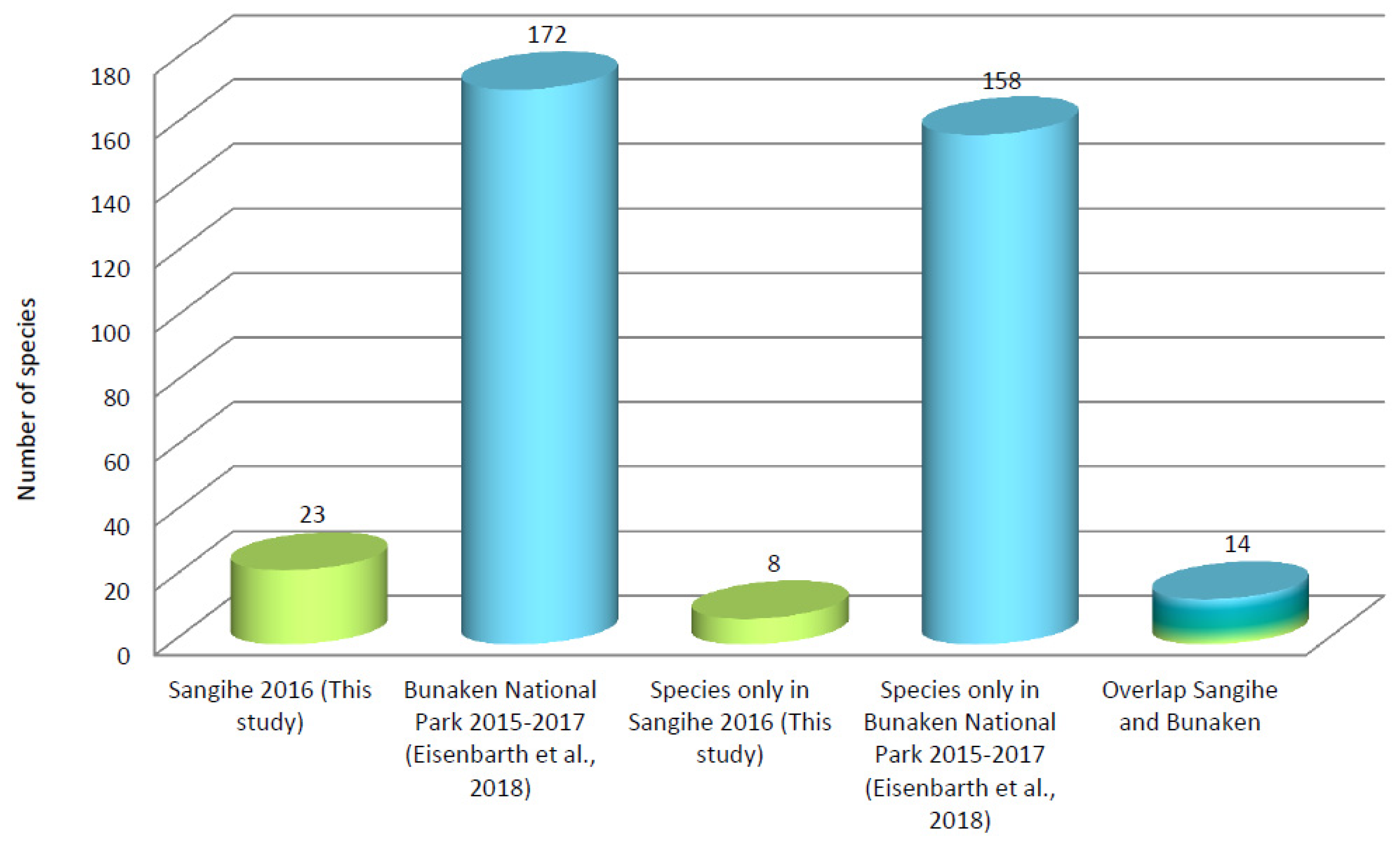
| Sangihe 2016 | 0 | 0 | 0 | 3 | 0 | 0 | 19 | 1 | 23 | This Study |
| BNP 2015–2017 | 0 | 24 | 4 | 26 | 0 | 2 | 69 | 47 | 172 | [14] |
| Ambon | 0 | 11 | 6 | 12 | 0 | 4 | 90 | 15 | 138 | [27,29,80] |
| Bali and Indonesia | 3 | 12 | 7 | 11 | 0 | 9 | 128 | 35 | 205 | [81] |
| Vietnam | 0 | 11 | 7 | 6 | 1 | 6 | 95 | 25 | 151 | [82] |
| Papua New Guinea | 0 | 71 | 9 | 61 | 0 | 8 | 257 | 132 | 538 | [83] |
| Taiwan | 0 | 2 | 0 | 4 | 0 | 1 | 53 | 10 | 70 | [84] |
| Hong Kong | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 14 | 54 | [85] |
| Chagos Archipelago | 0 | 2 | 1 | 2 | 0 | 0 | 30 | 6 | 41 | [86] |
| Maldives | 0 | 4 | 2 | 2 | 0 | 2 | 21 | 4 | 35 | [25] |
| Marshall Islands | 5 | 13 | 5 | 10 | 0 | 1 | 53 | 14 | 101 | [87] |
| Lizard Island | 4 | 28 | 6 | 21 | 0 | 4 | 66 | 29 | 158 | [88] |
| Mauritius | 0 | 5 | 5 | 0 | 0 | 2 | 22 | 1 | 35 | [77] |
| Western Australia | 7 | 22 | 12 | 21 | 2 | 6 | 115 | 31 | 215 | [89] |
| Fiji Islands | 10 | 30 | 6 | 26 | 1 | 6 | 127 | 45 | 251 | [90] |
| New Caledonia | 16 | 82 | 10 | 17 | 1 | 4 | 98 | 30 | 258 | [91] |
| Heron Island | 0 | 20 | 5 | 31 | 0 | 7 | 151 | 47 | 261 | [92] |
| Red Sea | 7 | 41 | 17 | 16 | 0 | 8 | 140 | 65 | 294 | [28] |
| Great Barrier Reef | 0 | 64 | 12 | 42 | 0 | 9 | 210 | 77 | 414 | [92] |
| Lakshadweep Islands | 1 | 6 | 5 | 9 | 0 | 4 | 27 | 8 | 60 | [93] |
| New Caledonia | 4 | 19 | 12 | 25 | 0 | 11 | 237 | 65 | 373 | [94] |
| New South Wales | 0 | 35 | 17 | 27 | 2 | 12 | 209 | 80 | 378 | [95] |
| Tropical East Pacific | 0 | 89 | 13 | 30 | 0 | 11 | 131 | 125 | 399 | [96] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Undap, N.; Papu, A.; Schillo, D.; Ijong, F.G.; Kaligis, F.; Lepar, M.; Hertzer, C.; Böhringer, N.; König, G.M.; Schäberle, T.F.; et al. First Survey of Heterobranch Sea Slugs (Mollusca, Gastropoda) from the Island Sangihe, North Sulawesi, Indonesia. Diversity 2019, 11, 170. https://doi.org/10.3390/d11090170
Undap N, Papu A, Schillo D, Ijong FG, Kaligis F, Lepar M, Hertzer C, Böhringer N, König GM, Schäberle TF, et al. First Survey of Heterobranch Sea Slugs (Mollusca, Gastropoda) from the Island Sangihe, North Sulawesi, Indonesia. Diversity. 2019; 11(9):170. https://doi.org/10.3390/d11090170
Chicago/Turabian StyleUndap, Nani, Adelfia Papu, Dorothee Schillo, Frans Gruber Ijong, Fontje Kaligis, Meita Lepar, Cora Hertzer, Nils Böhringer, Gabriele M. König, Till F. Schäberle, and et al. 2019. "First Survey of Heterobranch Sea Slugs (Mollusca, Gastropoda) from the Island Sangihe, North Sulawesi, Indonesia" Diversity 11, no. 9: 170. https://doi.org/10.3390/d11090170
APA StyleUndap, N., Papu, A., Schillo, D., Ijong, F. G., Kaligis, F., Lepar, M., Hertzer, C., Böhringer, N., König, G. M., Schäberle, T. F., & Wägele, H. (2019). First Survey of Heterobranch Sea Slugs (Mollusca, Gastropoda) from the Island Sangihe, North Sulawesi, Indonesia. Diversity, 11(9), 170. https://doi.org/10.3390/d11090170







