Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling
2.3. Sample Analysis
2.4. Data and Statistical Analyses
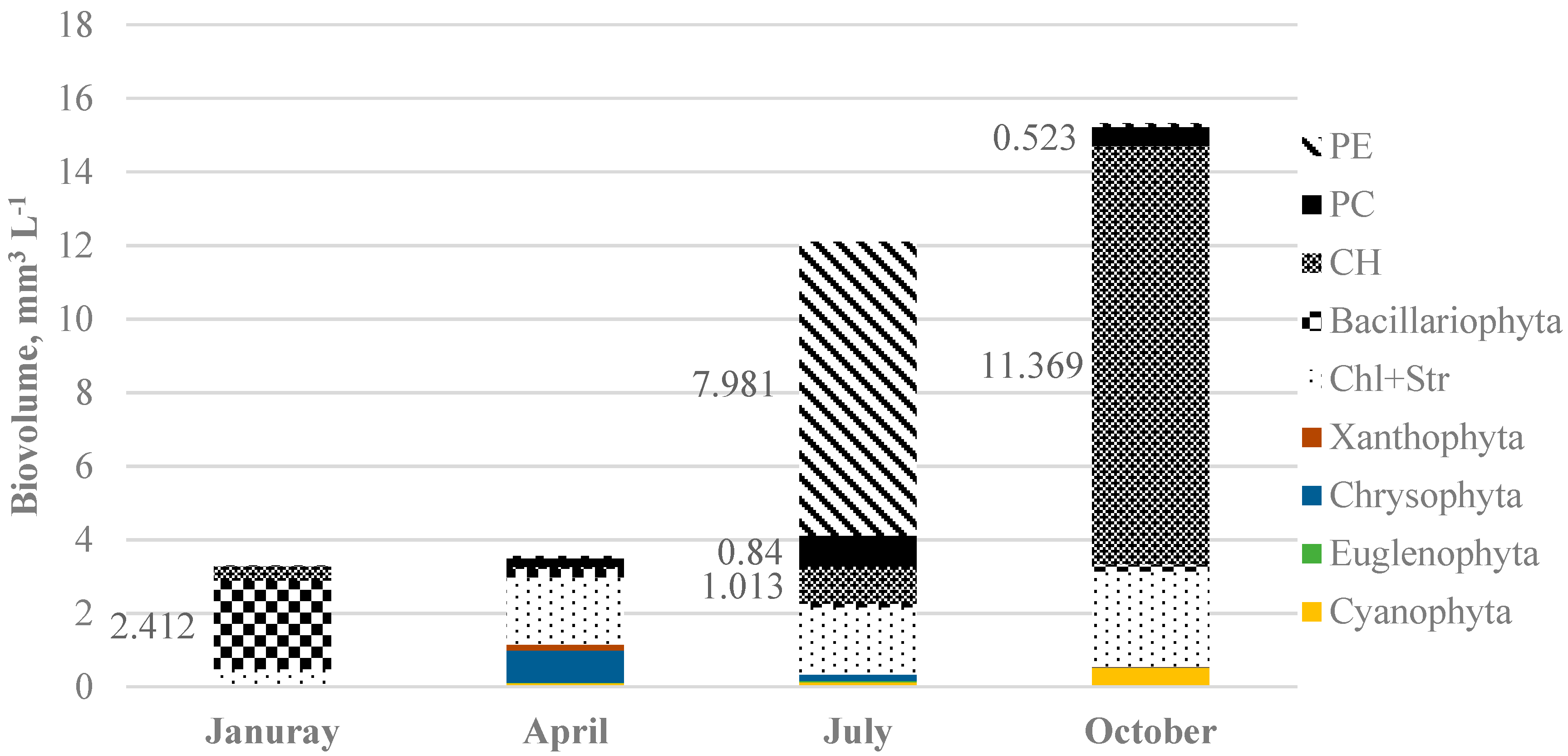
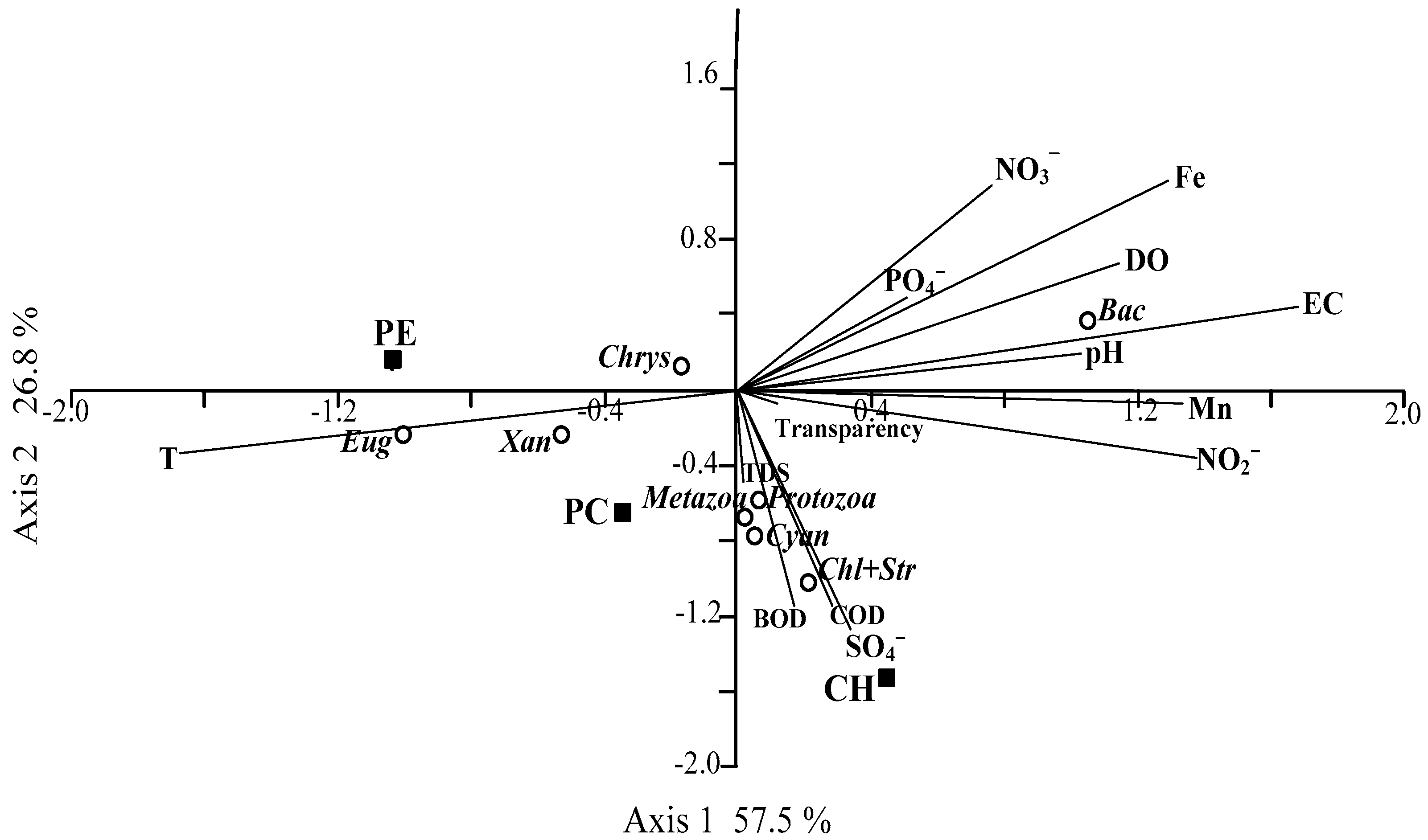
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirabayashi, K.; Yoshizawa, K.; Yoshida, N.; Ariizumi, K.; Kazama, F. Long-Term Dynamics of Freshwater Red Tide in Shallow Lake in Central Japan. Environ. Health Prev. Med. 2007, 12, 33–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yatigammana, S.K.; Ileperuma, O.A.; Perera, M.B.U. Water pollution due to a harmful algal bloom: A preliminary study from two drinking water reservoirs in Kandy, Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2011, 39, 91–94. [Google Scholar] [CrossRef]
- Viner-Mozzini, Y.; Zohary, T.; Gasith, A. Dinoflagellate bloom development and collapse in Lake Kinneret: A sediment trap study. J. Plankton Res. 2003, 25, 591–602. [Google Scholar] [CrossRef]
- Rengefors, K.; Legrand, C. Toxicity in Peridinium aciculiferum an adaptive strategy to outcompete other winter phytoplankton. Limnol. Oceanogr. 2001, 46, 1990–1997. [Google Scholar] [CrossRef]
- Regel, R.H.; Brookes, J.D.; Ganf, G.G. Vertical migration, entrainment and photosynthesis of the freshwater dinoflagellate Peridinium cinctum in a shallow urban lake. J. Plankton Res. 2004, 26, 143–157. [Google Scholar] [CrossRef]
- Margalef, R.; Mir, M.; Estrada, M. Phytoplankton composition and distribution as an expression of properties of reservoirs. Can. Water Res. J. 1982, 7, 26–50. [Google Scholar] [CrossRef]
- Padisak, J.; Hajnal, E.; Naselli-flores, L.; Dokulil, M.T.; Noges, P.; Zohary, T. Convergence and divergence in organization of phytoplankton communities under various regimes of physical and biological control. Hydrobiologia 2010, 639, 205–220. [Google Scholar] [CrossRef]
- Lopez, N.L.; Rondon, C.A.R.; Zapata, A.; Jimenez, J.; Vilamil, W.; Arenas, G.; Rincon, C.; Sanchez, T. Factors controlling phytoplankton in tropical high-mountain drinking-water reservoirs. Limnetica 2012, 31, 305–322. [Google Scholar]
- Agrawal, A.A. Algal defense, grazers, and their interactions in aquatic trophic cascades. Acta Oecol. 1998, 19, 331–337. [Google Scholar] [CrossRef]
- Heaney, S.I.; Talling, J.F. Ceratium hirundinella—Ecology of a complex, mobile and successful plant. In Forty-Eighth Annual Report for the Year Ended 31 March 1980; Freshwater Biological Association: Ambleside, UK, 1980; pp. 27–40. [Google Scholar]
- Whittington, J.; Sherman, B.; Green, D.; Oliver, R.L. Growth of Ceratium hirundinella in a subtropical Australian reservoir: The role of vertical migration. J. Plankton Res. 2000, 22, 1025–1045. [Google Scholar] [CrossRef]
- Kawabata, Z.; Kagawa, H. Distribution pattern of the dinoflagellate Ceratium hirundinella (O. F. Müller) Bergh in a reservoir. Hydrobiologia 1988, 169, 319–325. [Google Scholar] [CrossRef]
- Sigee, D.C.; Levado, E.; Dodwell, A.J. Elemental composition of depth samples of Ceratium hirundinella (Pyrrophyta) within a stratified lake: An X-ray microanalytical study. Aquat. Microb. Ecol. 1999, 19, 177–187. [Google Scholar] [CrossRef][Green Version]
- Perez-Martınez, C.; Sanchez-Castillo, P. Temporal occurrence of Ceratium hirundinella in Spanish reservoirs. Hydrobiologia 2001, 452, 101–107. [Google Scholar] [CrossRef]
- Hedger, R.D.; Olsen, N.R.B.; George, D.G.; Malthus, T.J.; Atkinson, P.M. Modelling spatial distributions of Ceratium hirundnella and Microcystis spp. in a small productive British lake. Hydrobiologia 2004, 528, 217–227. [Google Scholar] [CrossRef]
- MacDonagh, M.E.; Casco, M.A.; Claps, M.C. Colonization of a Neotropical Reservoir (Córdoba, Argentina) by Ceratium hirundinella (O. F. Müller) Bergh. Ann. Limnol. Int. J. Limnol. 2005, 41, 291–299. [Google Scholar] [CrossRef]
- Hart, R.C.; Wragg, P.D. Recent blooms of the dinoflagellate Ceratium in Albert Falls Dam (KZN): History, causes, spatial features and impacts on a reservoir ecosystem and its zooplankton. Water SA 2009, 35, 455–468. [Google Scholar] [CrossRef]
- Bruno, S.F.; McLaughlin, J.J.A. The nutrition of the freshwater dinoflagellate Ceratium hirundinella. J. Protozool. 1977, 24, 548–553. [Google Scholar] [CrossRef]
- Pfiester, L.A. Periodicity of Ceratium hirundinella (O.F.M.) Dujardin and Peridinium cinctum (O.F.M.) Ehrenberg in relation to certain ecological factors. Castanea 1971, 36, 246–257. [Google Scholar]
- Grigorszky, I.; Kiss, K.T.; Béres, V. The effects of temperature, nitrogen, and phosphorus on the encystment of Peridinium cinctum, Stein (Dinophyta). Hydrobiologia 2006, 563, 527–535. [Google Scholar] [CrossRef]
- Belkinova, D.; Padisák, J.; Gecheva, G.; Cheshmedjiev, S. Phytoplankton based assessment of ecological status of Bulgarian lakes and comparison of metrics within the water framework directive. Appl. Ecol. Environ. Res. 2014, 12, 83–103. [Google Scholar] [CrossRef]
- Pollinger, B.U.; Hickel, B. Dinoflagellata associations in subtropical lake. Arch. Hydrobiol. 1991, 120, 267–285. [Google Scholar]
- Popovsky, J. Some thecate Dinoflagellates from Cuba. Arch. Protistenkd. 1970, 112, 252–258. [Google Scholar]
- Krakhmalniy, A. Morphology of Peridiniopsis elpatiewskyi (Ostenf.) Bourr. (Dinophyta) theca. Int. J. Algae 2009, 11, 25–33. [Google Scholar] [CrossRef]
- Katsiapi, M. Assessing Water Quality of Greek Lakes and Reservoirs Using Ecological and Molecular Markers. Ph.D. Thesis, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2012; p. 148. [Google Scholar]
- Täuscher, L. Checklisten und Gefährdungsgrade der Algen des Landes Brandenburg. Verh. Bot. Ver. Berl. Brandenbg. 2013, 146, 109–128. [Google Scholar]
- Ascencio, E.; Rivera, P.; Cruces, F. Morphology of Peridiniopsis elpatiewskyi (Ostenfeld) Bourrelly (Dinophyceae) found for the first time in Chilean inland waters. Gayana Bot. 2015, 72, 42–46. [Google Scholar] [CrossRef]
- Pourmoghaddas, H. Water Quality and Health Issues in the Zayandeh Rud Basin; International Water Management Institute: Isfahan, Iran, 2006; p. 30. [Google Scholar]
- Shahmansuri, M. Investigation of Trophic State and Thermal Stratification of Reservoir of Zayandeh Rood; Regional Water Company of Esfahan: Isfahan, Iran, 2005; p. 159. [Google Scholar]
- Hoseiniabri, H. Zayandeh Rud River from Headwaters to Wetland; Golha Publication: Isfahan, Iran, 1996; p. 91. [Google Scholar]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 2008; p. 293. [Google Scholar]
- Pechenik, J.A. Biology of the Invertebrates; Wm. C. Brown Publishers: Dubuque, IA, USA, 1991; p. 567. [Google Scholar]
- Wasser, S.P.; Kondrateva, N.V.; Masyuk, N.P.; Palamar-Mordvintseva, G.M. Algae: Reference Book; Wasser, S.P., Kondrateva, N.V., Masyuk, N.P., Palamar-Mordvintseva, G.M., Eds.; Nauk. Dumka: Kiev, Ukraine, 1989; p. 608. (In Russian) [Google Scholar]
- Fritz, L.; Triemer, R.E. A rapid simple technique utilizing Calcofluor White M2R for the visualization of Dinoflagellate thecal plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Lange-Bertalot, H. Diatoms of Europe: Diatoms of the European inland waters and comparable habitats. In Navicula Sensu Stricto. 10 Genera Separated from Navicula Sensu Lato. Frustulia.; A.R.G. Gantner Verlag K.G.: Ruggell, Lichtenstein, 2001; Volume 2, p. 526. [Google Scholar]
- Proshkina-Lavrenko, A.I. Diatoms of the USSR: Fossil and Recent; Nauka Press: Leningrad, Russia, 1974; p. 40. (In Russian) [Google Scholar]
- Parker, T.J.; Haswell, W.A. Textbook of Zoology: Invertebrates; Macmillan: London, UK, 1972; p. 874. [Google Scholar]
- Barnes, R.S.K.; Calow, P.; Olive, P.J.W.; Golding, D.W.; Spicer, J.I. The Invertebrates: A Synthesis, 3rd ed.; Wiley-Blackwell Publisher: Hoboken, NJ, USA, 2001; p. 512. [Google Scholar]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Vadrucci, M.; Mazziotti, C.; Fiocca, A. Cell biovolume and surface area in phytoplankton of Mediterranean transitional water ecosystems: Methodological aspects. Transit. Waters Bull. 2013, 7, 100–123. [Google Scholar]
- Perez-Martınez, C.; Sanchez-Castillo, P. Winter dominance of Ceratium hirundinella in a southern north-temperate reservoir. J. Plankton Res. 2002, 24, 89–96. [Google Scholar] [CrossRef]
- Prioretti, L.; Gontero, B.; Hell, R.; Giordano, M. Diversity and regulation of ATP sulfurylase in photosynthetic organisms. Front. Plant Sci. Front. 2014, 5, 597. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Prioretti, L. Sulphur and Algae: Metabolism, Ecology and Evolution. In The Physiology of Microalgae; Borowitzka, M.A., Beardall, J., Raven, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 185–209. [Google Scholar]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef] [PubMed]
- Hinga, K.R. CO-occurrence of dinoflagellate blooms and high pH in marine enclosures. Mar. Ecol. Prog. Ser. 1992, 86, 181–187. [Google Scholar] [CrossRef]
- Coria-Monter, E.; Monreal-Gomez, M.A.; Salas-de-Leon, D.A.; Aldeco-Ramırez, J.; Merino-Ibarra, M. Differential distribution of diatoms and dinoflagellates in a cyclonic eddy confined in the Bay of La Paz, Gulf of California. J. Geophys. Res. Oceans 2014, 119, 6258–6268. [Google Scholar] [CrossRef]
- Duarte, P.; Macedo, M.F.; Cancela da Fonseca, L. The relationship between phytoplankton diversity and community function in a coastal lagoon. In Marine Biodiversity: Patterns and Processes, Assessment, Threats, Management and Conservation, Proceedings of the 38th European Marine Biology Symposium, Aveiro, Portugal, 8–12 September 2003; Queiroga, H., Cunha, M.R., Cunha, A., Moreira, M.H., Quintino, V., Rodrigues, A.M., Serodio, J., Warwick, R.M., Eds.; Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 2006; Volume 183, pp. 3–18. [Google Scholar]
- Smayda, T.J. Adaptive Ecology, Growth Strategies and the Global Bloom Expansion of Dinoflagellates. J. Oceanogr. 2002, 5, 281–294. [Google Scholar] [CrossRef]
- Huntley, M.; Sykes, P.; Rohan, S.; Marin, V. Chemically-mediated rejection of dinoflagellate prey by the copepods Calanus pacificus and Paracalanus parvus: Mechanism, occurrence and significance. Mar. Ecol. Prog. Ser. 1986, 28, 105–120. [Google Scholar] [CrossRef]
- Spataru, P. The feeding habits of Tilapia galilaea (Artedi) in Lake Kinneret (Israel). Aquaculture 1976, 9, 47–59. [Google Scholar] [CrossRef]
- Darki, B.Z.; Darki, L.Z.; Akkafi, H.R.; Mirzai, M. Taxonomic composition of algae and its indicator role in the ecosystem of the Zayandeh Rud River, Iran. Inland Water Biol. 2013, 6, 285–293. [Google Scholar] [CrossRef]
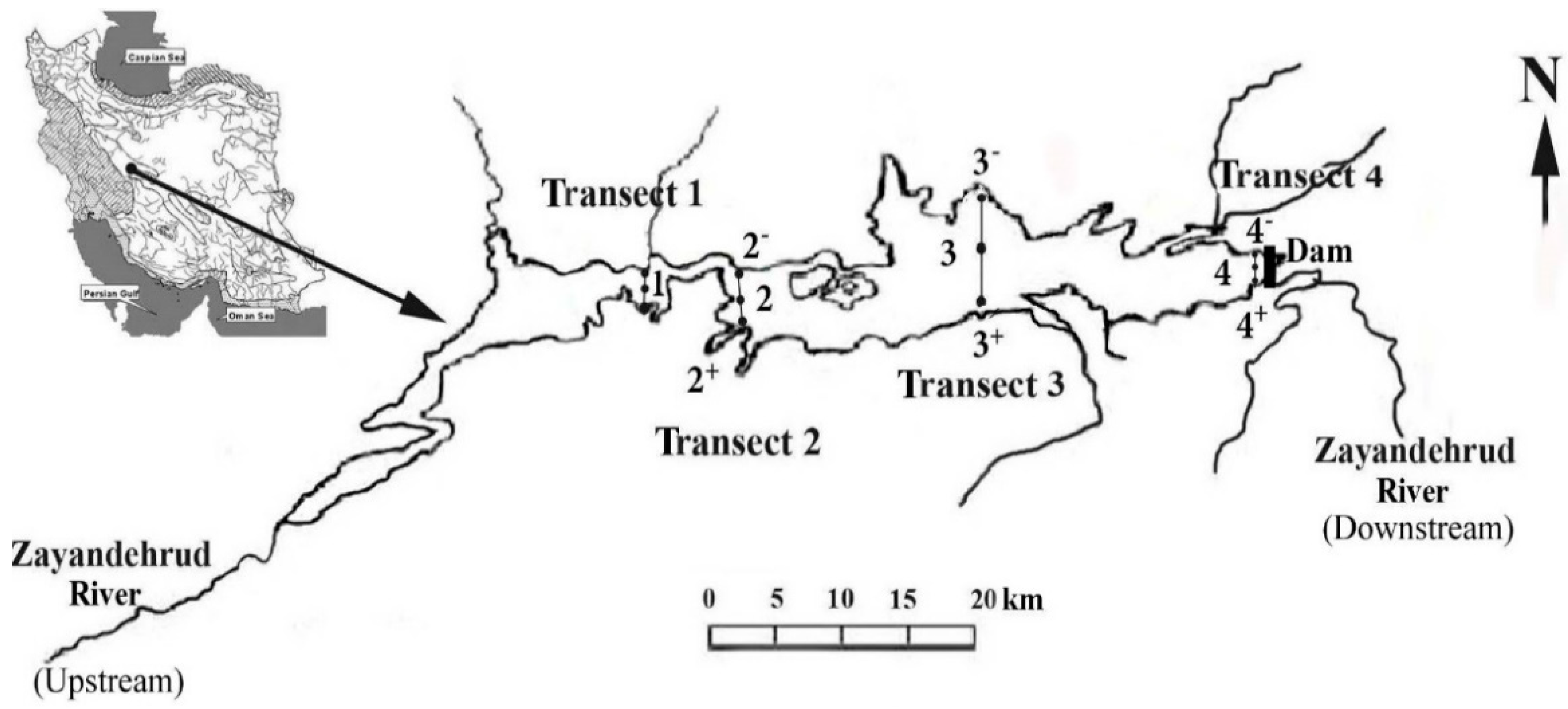
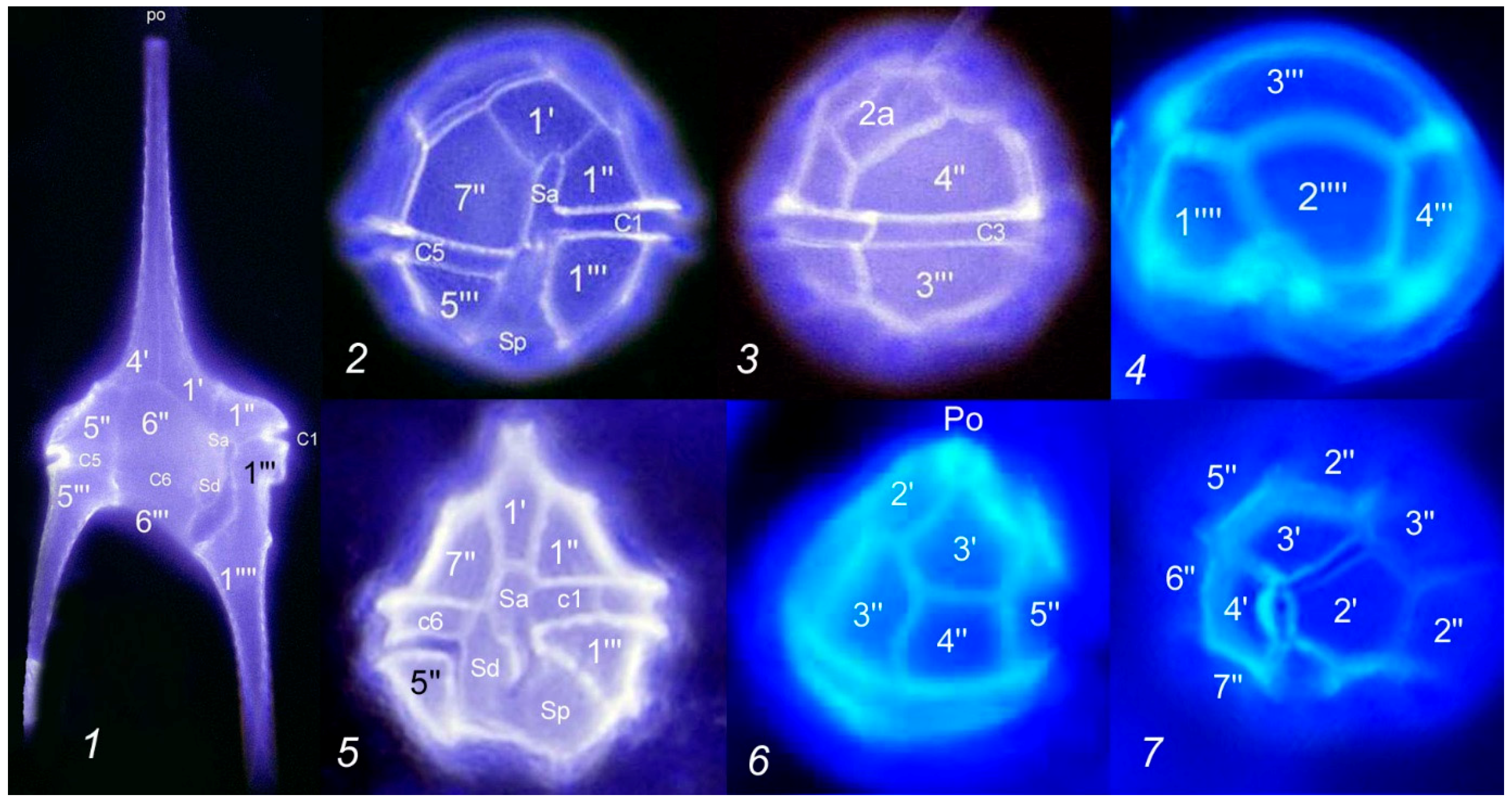
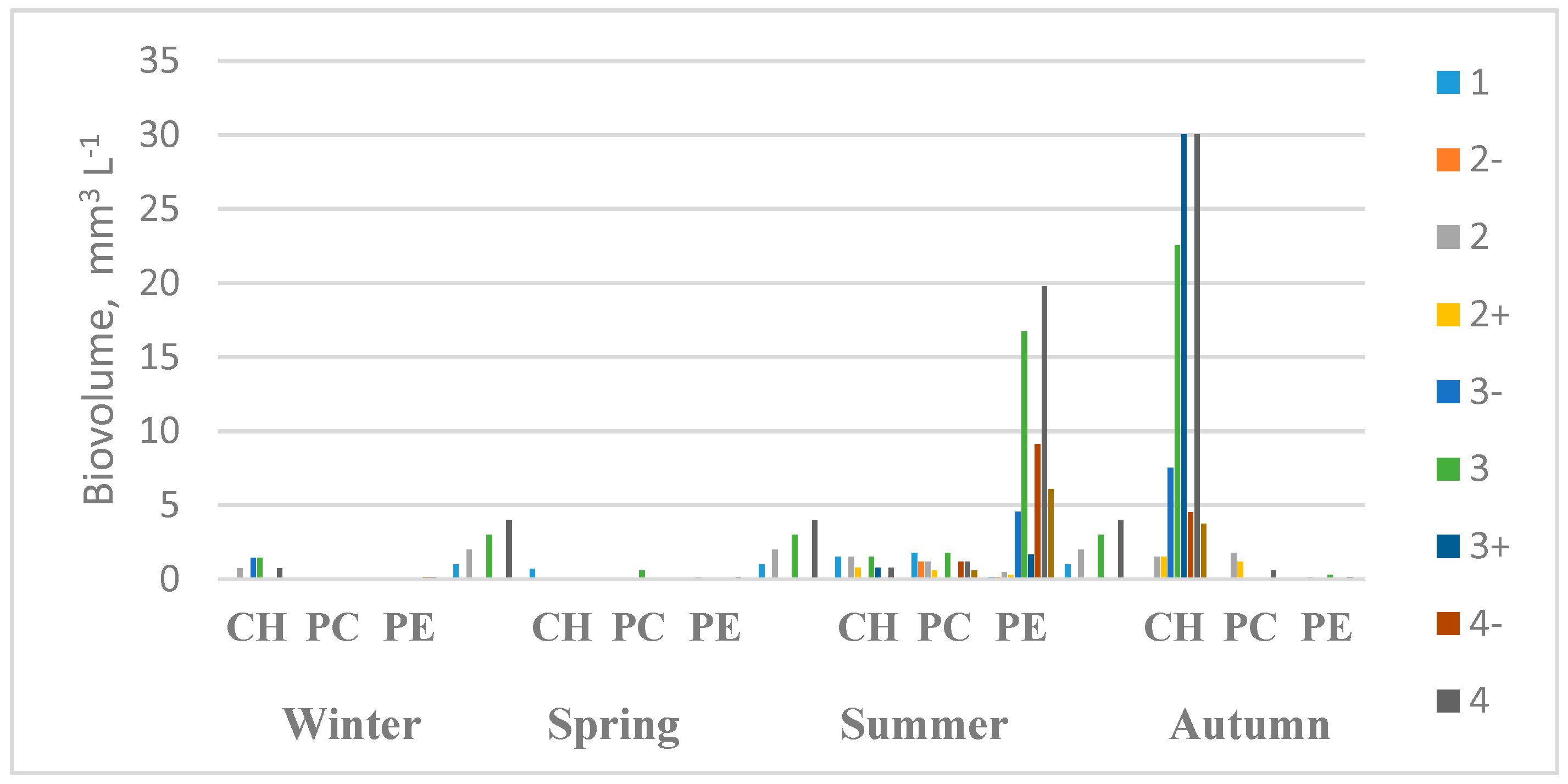
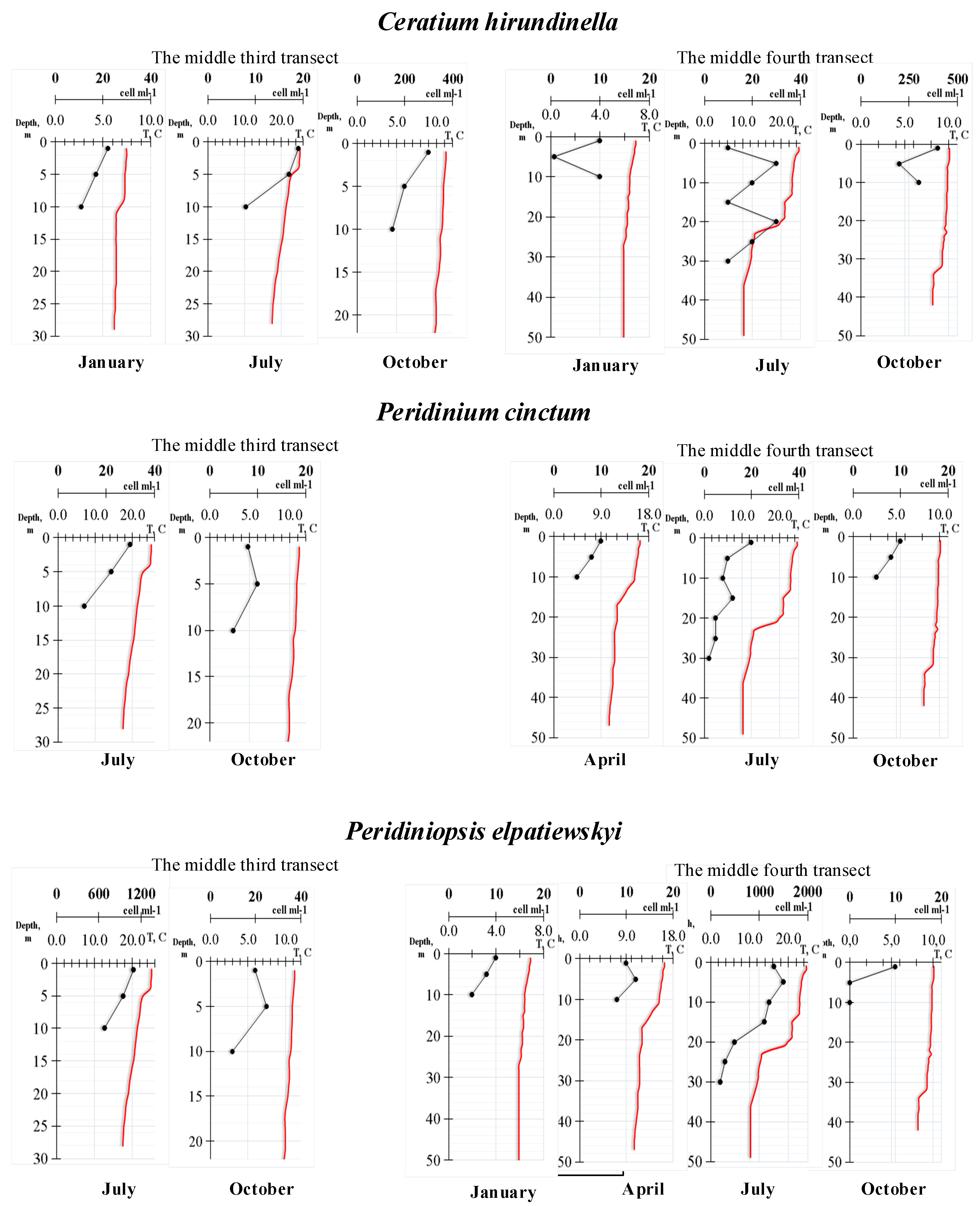
| Transects | Geographic Coordinates | Depth (m) | |||
|---|---|---|---|---|---|
| January | April | July | October | ||
| 1 | 50°31’45.74” E–32°42’56.38” N | 1.2 | 1.4 | 1.5 | 1.0 |
| 2 | 50°36’31.58” E–32°43’09.32” N | 10.5 | 7 | 8.3 | 5.5 |
| 3 | 50°39’56.68” E–32°44’08.97” N | 32 | 27 | 28.4 | 23.5 |
| 4 | 50°44’02.37” E–32°44’01.72” N | 51 | 48 | 49.3 | 43 |
| Abiotic and Biotic Variables | Species | |||||
|---|---|---|---|---|---|---|
| CH | PC | PE | ||||
| Min–Max | Mean ± S.D | Min–Max | Mean ± S. D | Min–Max | Mean ± S.D | |
| Transparency (m) | 0.3–6.2 | 3.3 ± 1.76 | 2.8–4.2 | 3.6 ± 0.57 | 0.68–6.2 | 3.25 ± 1.83 |
| TDS (ppm) | 150–260 | 191.1 ± 37.21 | 152–262 | 195.8 ± 41.4 | 152–262 | 192.2 ± 35.9 |
| pH | 7.4–8.7 | 8.04 ± 0.44 | 6.7–8.4 | 7.9 ± 0.46 | 6.7–8.7 | 8.02 ± 0.45 |
| Temperature (T, °C) | 6.4–25.6 | 14.8 ± 5.6 | 11–25.6 | 17.5 ± 4.9 | 11–25.6 | 15.06 ± 5.69 |
| EC (μS cm−1) | 230–413 | 329.5 ± 66.09 | 230–413 | 295 ± 43.26 | 230–413 | 324.6 ± 64.2 |
| DO (mg L−1) | 5.2–8.3 | 7.8 ± 1.35 | 5.2–8.3 | 7.1 ± 0.91 | 5.2–8.3 | 7.7 ± 1.24 |
| NO3− (mg L−1) | 3.8–12.6 | 9.8 ± 4.97 | 3.8–12.6 | 8.02 ± 3.12 | 3.8–12.6 | 9.64 ± 4.2 |
| NO2− (mg L−1) | 0.055–0.15 | 0.07 ± 0.03 | 0.052–0.11 | 0.06 ± 0.03 | 0.052–0.11 | 0.06 ± 0.03 |
| PO4− (mg L−1) | 0.02–0.17 | 0.06 ± 0.03 | 0.02–0.17 | 0.06 ± 0.03 | 0.02–0.17 | 0.06 ± 0.03 |
| SO4− (mg L−1) | 14.6–236.6 | 46.2 ± 49.3 | 14.6–236.6 | 55.6 ± 56.4 | 14.6–236.6 | 44.3 ± 50.05 |
| Fe (mg L−1) | 0.002–0.2 | 0.06 ± 0.06 | 0.002–0.10 | 0.025 ± 0.038 | 0.002–0.15 | 0.06 ± 0.06 |
| Mn (mg L−1) | 0.003–0.006 | 0.003 ± 0.002 | 0.001–0.006 | 0.002 ± 0.001 | 0.001–0.006 | 0.003 ± 0.002 |
| COD (mg L−1) | 2–57 | 12.24 ± 12.72 | 2–57 | 14.3 ± 13.6 | 2–57 | 11.7 ± 12.79 |
| BOD (mg L−1) | 1–29 | 14.25 ± 14.59 | 1–29 | 7.53 ± 6.5 | 1–29 | 6.24 ± 5.79 |
| Species | Water Body | Max Cell Number, Cells mL−1 | Depth | Period of Time | Authors |
|---|---|---|---|---|---|
| CH | Zayandeh Rud Reservoir, Iran | 400 | Surface | In October | Current study |
| Albert Falls Dam (South Africa) | 5000 | - | In October | Hart and Wragg [17] | |
| The Ishitegawa Reservoir (Japan) | 1300 | Surface | In July | Kawabata, Kagawa [12] | |
| Rio Tercero Reservoir | 1244 | Photic zone | later summer | MacDonagh et al. [16] | |
| Esthaite Water (England) | 1000 | In the 0–5 m layer | In September | Heaney and Talling [10] | |
| Bermejales Reservoir (Spain) | 247 | - | In December | Perez-Martınez, Sanchez-Castillo [14] | |
| Lake Kinneret (Israel) | 35 | Photic zone | in May | Pollinger, Hickel [22] | |
| PC | Zayandeh Rud Reservoir, Iran | 50 | At the bottom (5.5 m) | In October | Current study |
| Torrens Lake (South Australia) | 460 | Below the surface mixed layer | In January (summer month) | Regel et al. [5] | |
| Man-made lake near Tokyo | 420 | - | - | Pollinger, Hickel [22] | |
| PE | Zayandeh Rud Reservoir, Iran | 1500 | At a 5 m depth | In July | Current study |
| Lake Kinneret (Israel) | 100 | Near the surface | In summer | Pollinger, Hickel [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei Darki, B.; Krakhmalnyi, A.F. Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi. Diversity 2019, 11, 137. https://doi.org/10.3390/d11080137
Zarei Darki B, Krakhmalnyi AF. Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi. Diversity. 2019; 11(8):137. https://doi.org/10.3390/d11080137
Chicago/Turabian StyleZarei Darki, Behrouz, and Alexandr F. Krakhmalnyi. 2019. "Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi" Diversity 11, no. 8: 137. https://doi.org/10.3390/d11080137
APA StyleZarei Darki, B., & Krakhmalnyi, A. F. (2019). Biotic and Abiotic Factors Affecting the Population Dynamics of Ceratium hirundinella, Peridinium cinctum, and Peridiniopsis elpatiewskyi. Diversity, 11(8), 137. https://doi.org/10.3390/d11080137




