1. Introduction
The growing impact of human-induced changes on natural ecosystems, such as land transformation and habitat degradation, is leading to the pressing need for straightforward methodologies for monitoring biodiversity in space and time [
1,
2,
3,
4,
5]. Although broad-scale patterns of biodiversity are well documented, accurate descriptions of the distribution of biodiversity which down at fine spatial, temporal, or taxonomic scales are still missing, even for well-described groups, such as vascular plants or vertebrates [
6].
Surrogacy can be defined as the relationship existing between a surrogate variable and an “objective” variable (also called “target variable” [
7]). In ecology, cross-taxon congruence analysis can be expressed as the correlation in patterns of species richness and/or diversity [
8] or, in a multi-species context, as community concordance (i.e., the relationship among compositional patterns of multiple taxonomic groups across sites [
9,
10]). More in general, cross-taxon congruence occurs when diversity and/or composition patterns of different biological groups covary spatially [
11]. The interest in biological surrogates during the last decade has resulted in an increasing number of studies testing their effectiveness, in a multiplicity of locations and at different spatial scales [
12]. Rodrigues and Brooks [
13] pointed out that the use of surrogate taxa in conservation planning is substantially more effective than that of surrogates based on environmental data only. However, the effectiveness of the use of one taxon to predict community patterns for other taxonomic groups ultimately depends on its underlying mechanisms and on the strength of the relationship with, and among, such groups (e.g., [
14,
15,
16,
17,
18]). Furthermore, the effectiveness of surrogate taxa as ecological indicators for biodiversity assessment also depends on other factors, such as the spatial scale of analysis and the choice of predictor variables [
19]. The choice of the study scale is, in fact, also crucial to avoid spurious or undetected relationships among the collected variables and it could influence the time/cost of the sampling effort as well [
20].
From an ecological perspective, the factors that affect cross-taxa relationships include the following: (1) a similar but independent response from two taxonomic groups to the same set of environmental conditions [
9,
21,
22], (2) trophic interactions or functional interdependence [
9], (3) a shared bio-geographical and evolutionary history at a large/global scale [
23], and (4) species–energy relationships (e.g., [
3]; for a summary see [
24,
25]). Thus, potential surrogate taxa should have the following properties [
26,
27]: (i) a well-known and stable taxonomy so that populations can be defined in a reliable way; (ii) a well understood biology and general life history; (iii) occur over a broad geographical range and breadth of habitat types at higher taxonomic levels (order, family) so that results will be broadly applicable; (iv) specialization of each population (at lower taxonomic levels, e.g., species, subspecies), within a narrow habitat, which is likely to make them sensitive to habitat change; (v) some evidence that patterns observed in the surrogate taxon do replicate in other taxa, which are more difficult to investigate in the overall biodiversity at different spatial scales. Since vascular plants play a crucial role in land management, they can be a convenient choice as surrogate taxa. Furthermore, plants are fundamental structural and functional components of terrestrial ecosystems, having the major role in net primary productivity. Vascular plants are widely used for depicting biodiversity hotspots to address the institution of natural reserves, to identify priorities for conservation actions, and, more in general, for environmental planning [
28,
29,
30,
31]. Since their sampling is relatively easy [
32,
33,
34] and their taxonomy is sufficiently well described and standardized, they may reflect the diversity of other important, and less known and/or inconspicuous taxa, such as cryptogams.
Cryptogams, such as bryophytes and lichens, are rarely included in floristic and vegetation assessments for management and monitoring purposes due to difficulties encountered in their identification. Vascular plants are therefore of great interest to be used as a proxy for these groups of cryptogams. Some authors have tested the possible congruence between vascular plants and cryptogams for different habitats, locations, and spatial scales, but the results are fragmentary and conflicting [
35]. For instance, contrasting results were observed in several studies using vascular plants as a surrogate group for lichens in forest ecosystems [
36,
37,
38,
39]. Vascular plants proved to be effective surrogate taxa to select sites for conservation purposes, especially if used in combination with other factors [
37]. In contrast, another study showed that vascular plants can be ineffective as surrogate taxa for cryptogams [
38], even though this could be explained by an over simplification of the forest structure as a consequence of human management. Contrasting patterns were also observed in the Mediterranean area, even though only a few studies tested for the congruence between vascular plants and cryptogams [
19,
34,
40,
41]. These studies highlighted a limited effectiveness of cross-taxon estimates in a nature reserve in Tuscany, even though vascular plants may be useful surrogates of other organisms [
34,
42].
Despite the considerable amount of studies and meta-analyses on cross-taxon congruence [
12], few examples deal with freshwater ecosystems: to the best of our knowledge, the effectiveness of vascular plants as a surrogate group for lichens has never been specifically addressed in riparian freshwater communities in the Mediterranean area. Though Heino [
25] highlighted that cross-taxon congruence does not appear to be particularly relevant for conservation purposes in freshwater habitats, more recently Nascimbene et al. [
43] strengthened the importance of riparian woods for lichen conservation in riparian forests, providing important evidence of their role as hotspots of biodiversity [
44], as these fragile ecosystems are subjected to a high number of pressures and threats [
45].
In this study, then, we hypothesized that if the composition of lichen communities was consistently correlated to that of riparian vascular plants, the latter can be used as surrogate group when assessing lichen diversity of riparian habitats.
Since plants are generally easier to identify in the field than lichens, they could be efficiently used in preliminary and cost-effective biodiversity assessments. This would allow the collection of large-scale datasets on biodiversity and ecological indicators of the quality of river edges within a relatively short period of time, if compared to that required to survey and identify lichen taxa as well.
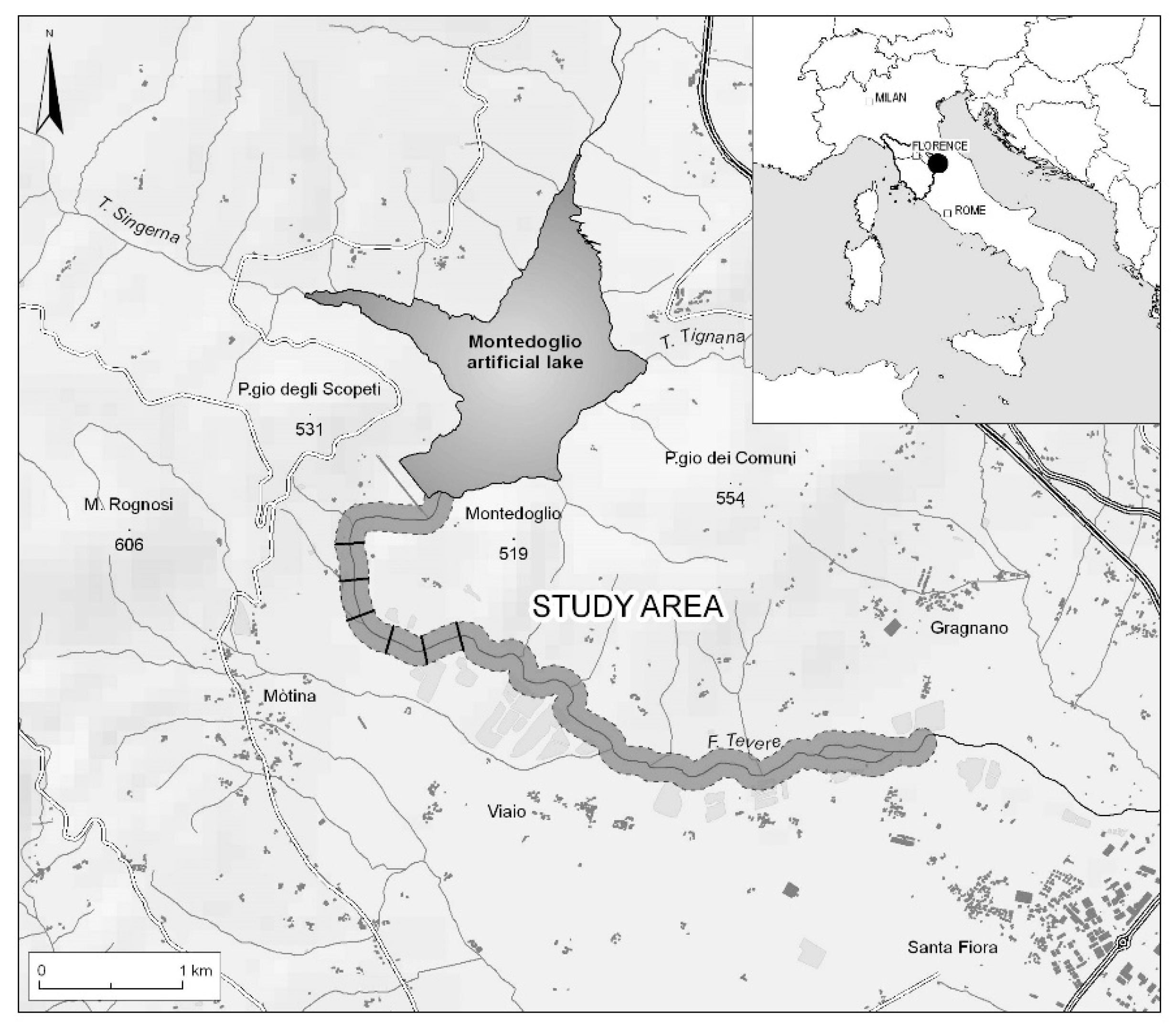
To assess whether plant communities can be a suitable surrogate group for lichen community composition and diversity, we surveyed vascular plants and lichens from five different habitats, located along a strong gradient of water flood, on a stretch of the Tiber river (Arezzo, central Italy). We aimed at the following: (1) assessing cross-taxon congruence in composition between lichen and plant communities, (2) quantifying the effectiveness of plant communities as surrogates of lichen communities, and (3) assessing if the degree of cross-taxon congruence is consistent along an environmental gradient in the riparian habitat. The predictive strength of vascular plants was evaluated using both species richness and species composition. Furthermore, the degree of cross-taxon congruence in species composition was assessed considering presence/absence and abundance data. To the best of our knowledge, this is the first in-depth analysis reporting the congruence in composition between plants and lichens in freshwater habitats which considers the variation of different parameters (data type, variation in environmental gradient, and scale of species abundances).
4. Discussion
The usefulness of surrogate taxon approaches is still controversial in ecological literature, with some studies showing strong cross-taxon congruence which is promising for their practical utilization, whereas others have found no congruence among taxa, limiting their use in conservation planning [
23,
70,
71,
72,
73,
74,
75]. In a recent study on pattern of congruence among taxa in European Temperate Forests, Burrascano et al. [
39] summarized the high variability in cross-taxon relationships and how the effect of spatial scale (grain and extent) could be pivotal for the observed variability. As an example, scarce relationships were observed between vascular plants and cryptogams in boreal forests [
76,
77], where bryophytes and lichens often constitute a major proportion of the species richness and biomass [
78,
79]. Disagreements in cross-taxon congruence are probably linked to differences among investigated studies in several key characteristics, such as the spatial scales, the study area location, and the analytical methods adopted [
19], on which the effectiveness of congruence among two or more taxa depend.
4.1. Congruence in Species Richness
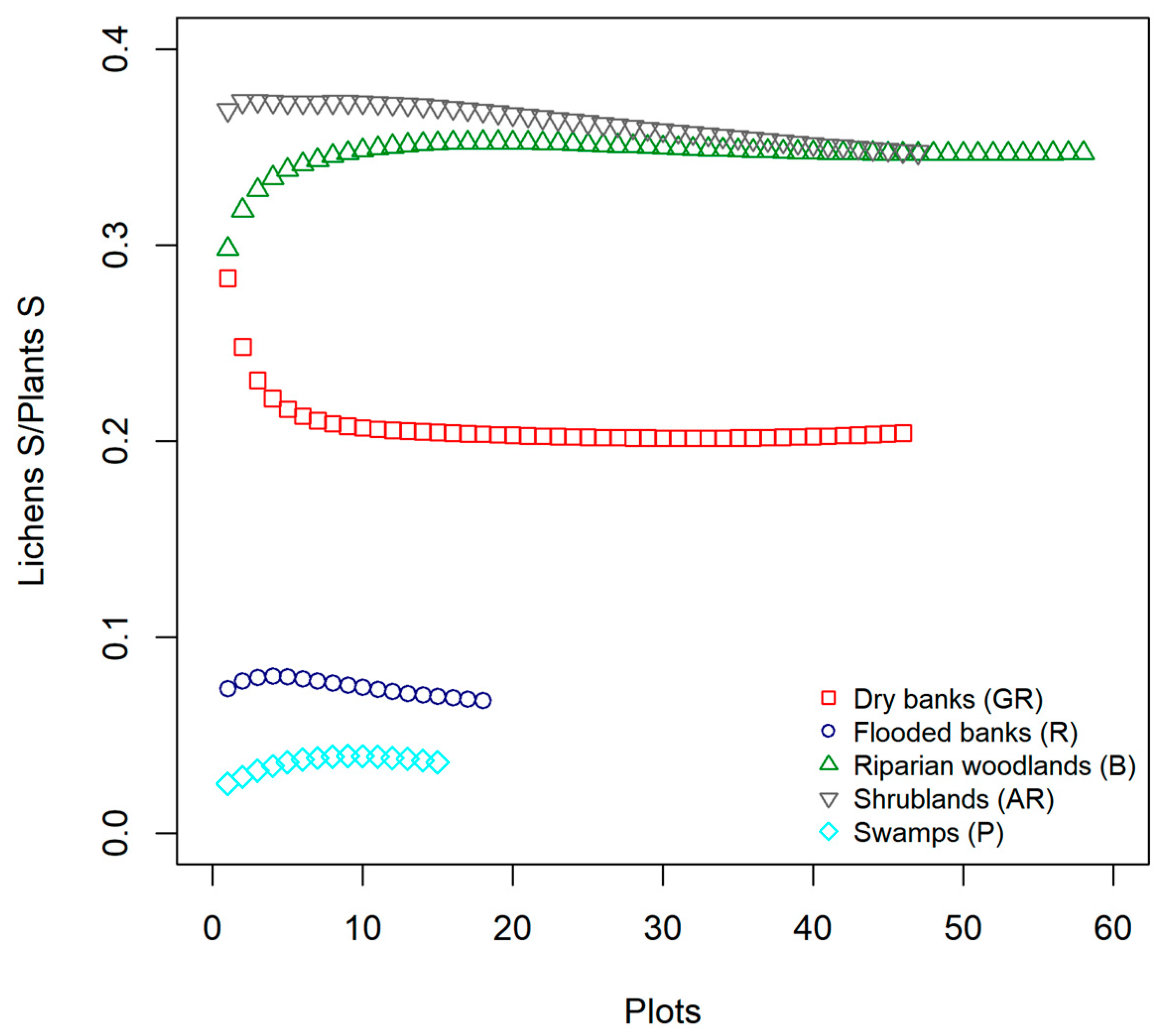
As pointed out by the cross-taxon correlation analysis we carried out between lichens and vascular plants richness, although the relationship resulted significant and positive in one of the five surveyed habitats (AR, shrublands) and negative in another one (P, Swamps), the overall performance of plant species richness per se as biodiversity surrogates of lichens was poor. Researchers working in various regions and using coarse grain plots (e.g., 50 × 50 m) obtained similar results and did not find any co-variation between the species richness of vascular plants and lichens [
36,
77]. In our study, many factors may have contributed to the general lack of species richness congruence and, among these, the small grain of the sampling units (1 m
2) may be considered one of them. Additionally, the functional characteristics of a particular taxon could be another factor affecting the degree of concordance among different taxonomic groups, along with the life history of the site. For instance, McMullin & Wiersma [
80] recently pointed out that the relative richness and abundance of lichens can be effective indicators of forest continuity and diversity. In this study, the surveyed habitats are riparian woods and shrublands, which are not characterized by mature stands. Though lichens can rely only on these trees and shrubs as stable substrata to develop their thalli and build communities richer in species abundance and diversity than in the other three habitat types, lichen diversity is still limited.
In assessing congruence patterns, species richness has been often used instead of species composition considering it is much simpler and faster to collect in the field. In surrogacy studies, it is often discussed whether it is reasonable to use species richness of vascular plants as a proxy of total biodiversity [
39,
42,
81,
82], even though different patterns of co-variation in lichen composition are described in relation to the variation of composition of plants communities. Our results also confirm the role of methodological issues, such as the type of data used (presence/absence vs. abundances) in determining the strength of cross-taxon relationships. Specifically, we observed that the degree of congruence in compositional patterns between lichens and vascular plants can substantially vary across habitats and depends on the type of the data used, along with the characteristic of the vegetation (abundance vs. presence/absence). Recent studies showed how the use of two-three taxa instead of a single one may drastically increase surrogacy [
83].
4.2. Congruence in Species Composition
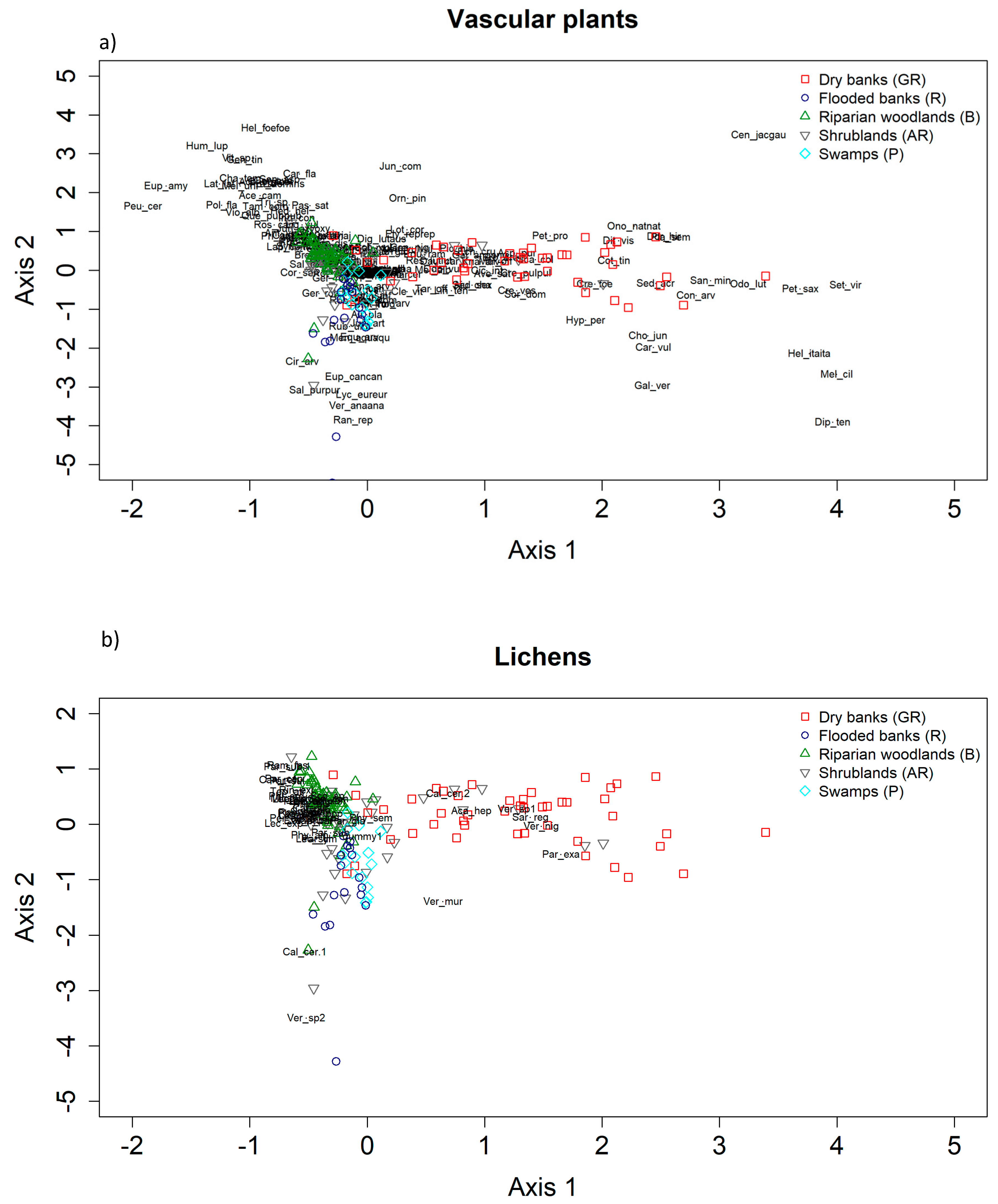
In general, our findings describe different patterns of co-variation in lichen composition in relation to the composition variation of plants communities. Our results also confirm the role of methodological issues such as the type of data used (presence/absence vs. abundances) in determining the strength of cross-taxon relationships. Specifically, we observed that the degree of congruence in compositional patterns between lichens and vascular plants can substantially vary across habitats and depends on the type of the data used, along with the characteristic of the vegetation (abundance vs. presence/absence data, species composition vs. variation in habitat characteristics). As for analysis at the species richness level discussed above, we showed a strong spatial structure of the data, which is also related to the spatial scale of the analysis. Specifically, at a coarser scale (i.e., along the ecological gradient), where environmental and structural gradients are more pronounced, especially for plant communities, these relationships display the strongest and clearest direction. On the other hand, at a finer scale, this signal seems to be hampered (see
Table 3).
Nonetheless, studies at smaller grains (e.g., 1 × 1 m) have shown relatively strong correlations between vascular plants and lichens (see, for instance [
71,
84]). In general, our results corroborate previous evidence, especially those concerning the covariation in plant and lichen composition along the whole transitional gradient from river-to-land. In fact, it is well-known that the configuration and heterogeneity of habitats (e.g., variation in habitat types) of an area strongly influences the number of species found in that area [
85]. Structurally complex and more mature habitats, indeed, provide more niches and diverse ways of exploiting the environmental resources, thus increasing species diversity [
86]. However, a weak degree of association in community composition of vascular plants and cryptogams was evidenced in other studies from Australian [
36], Canadian [
84], and New Zealand forests [
87] and dry grasslands in Sweden [
88]. In relation to our data, a previous study pointed out that the five habitats can be well defined and characterized using plant communities [
46]. The habitats host a specific set of plants, each with defined functional and structural features. Furthermore, our findings suggested a low compositional similarity, both among and within sampling units collected in the five habitats (see
Table 5 and
Table 6). Based on these marked compositional differences, results of the Co-CA highlighted clear ordination patterns, as follows: For vascular plants, the marshlands indicator species group (P) is constituted mostly of water-related taxa, such as hydrophytes (
Potamogeton nodosus), helophytes, or hygrophilous species (
Typha minima, Alisma plantago–aquatica, Epipactis palustris, Scirpoides holoschoenus, Lythrum salicaria, Lycopus europaeus). Here, the few lichen species which are present are not exclusive to this habitat and are not represented at all in the Co-CA regions identified by (P) plots. The indicator species of the flooded banks (R) are mostly plants requiring a high ground water content
(Mentha aquatica, Veronica anagallis–aquatica), species of cool and shaded habitats of the riparian forest fringes (
Petasites hybridus, Senecio aquaticus, Schedonorus giganteus), or species resistant to trampling (
Agrostis stolonifera, Prunella vulgaris). In this habitat, the recorded lichen species are those colonizing rocks, as the more stable substrate, and are mainly represented by the genus
Verrucaria, which is known to comprehend amphibian taxa of both fresh and salt water and to develop thin partially or completely endolithic thalli.
The floristic component characterizing the dry banks (GR) is instead mostly composed of xerophilous vascular plant species such as Bromus erectus, Sanguisorba minor, Ononis natrix, Plantago sempervirens, and Scabiosa columbaria. These species are not strictly linked to the presence of water and indicate habitual long emersion times, which clearly differentiates this type of environment. Due to the instability of the substrate, characterized by sandy soil and pebbles, and the lack of shrubs or trees as substrate, the lichen communities are species poor and only few epilithic taxa were mainly surveyed, such as Sargogye regularis and Verrucaria spp.
The indicator species of riparian woods (B) are mostly trees which characterize the physiognomy of this habitat (
Populus nigra and Alnus glutinosa) and a large number of shrubs, such as
Cornus sanguinea,
Ligustrum vulgare, Fraxinus ornus, and
Rubus caesius. The presence of the invasive alien species
Robinia pseudoacacia is also particularly significant [
46]. Here, instead, lichens are mainly represented by epiphytic species, among which the few more generalist, nitrophilous taxa, such as
Xanthoria parietina,
Lecidella elaeochroma, and
Lecanora hagenii, are frequently recorded.
A general consideration of the collected lichens relies on the fact that foliose macrolichens, such as those represented by the genera Parmelia and Ramalina, have been seldom surveyed across the five habitats. This might likely be due to the young forest/shrubs stands, in which the large foliose lichens did not have still time to develop conspicuously. The surveyed communities are mainly characterized by crustose species, which easily develop on the rather smooth bark of the tree and shrub species, such as those of the genera Lecanora, Lecidella, and Calopaca.
Finally, in our analysis we observed that the degree of cross-taxon congruence in species composition does not change strongly, regardless of the type of predictor variable used (abundance vs. presence/absence). Although it has been suggested that abundance data provide relatively detailed information concerning composition and structure of the communities [
19,
89], the collection of this type of data is labour and cost intensive. On other hand, the presence/absence data are less precise but much more cost effective. Our results showed that presence/absence data, for almost all the selected methodologies, provided similar results than those achieved by using abundances even if, on average, relationships were strongest when abundances were considered [
19,
90].