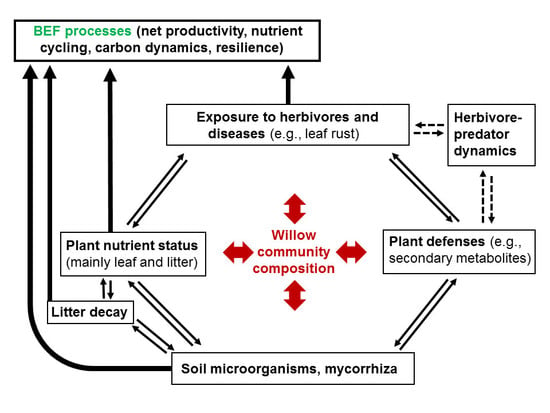
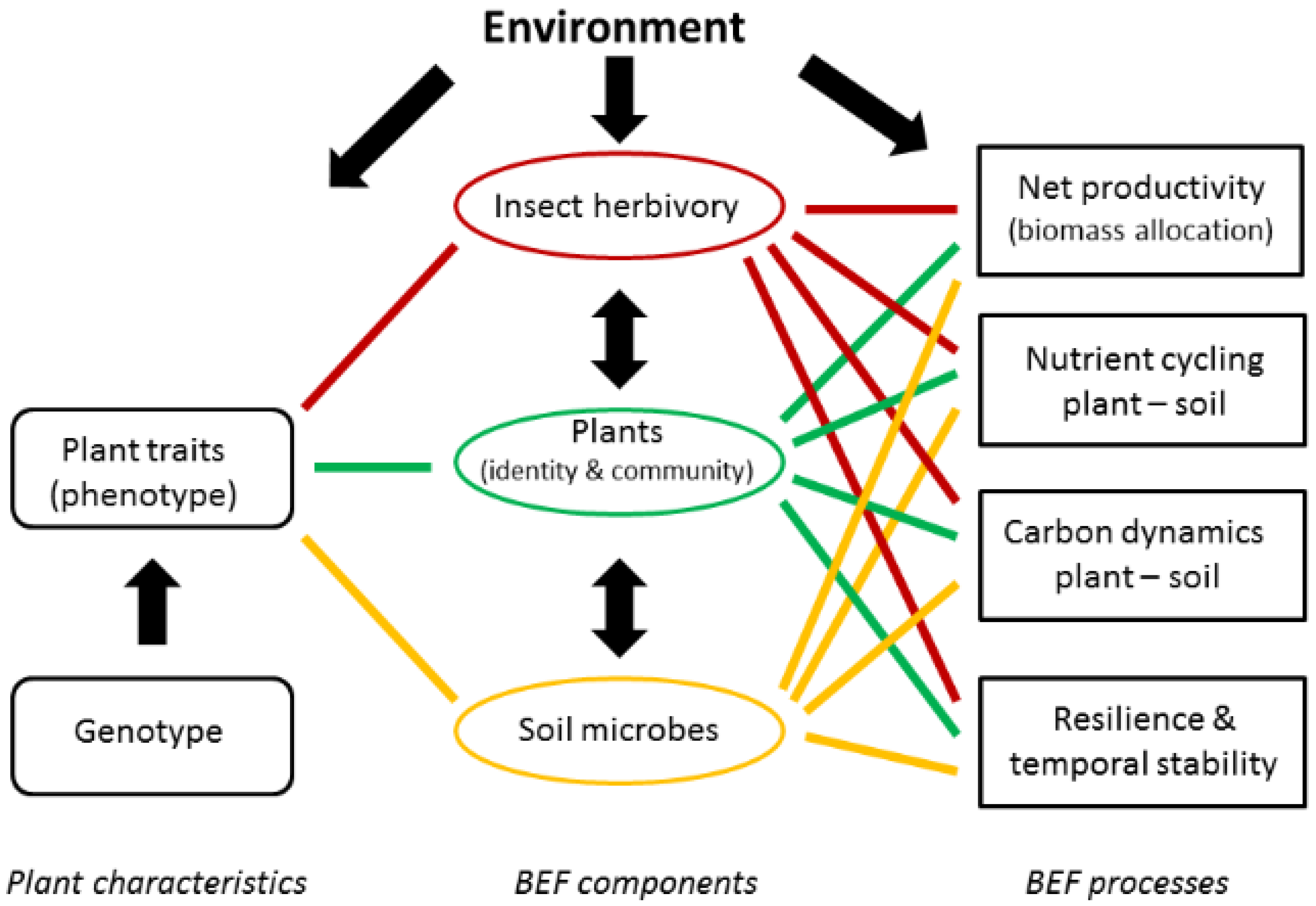
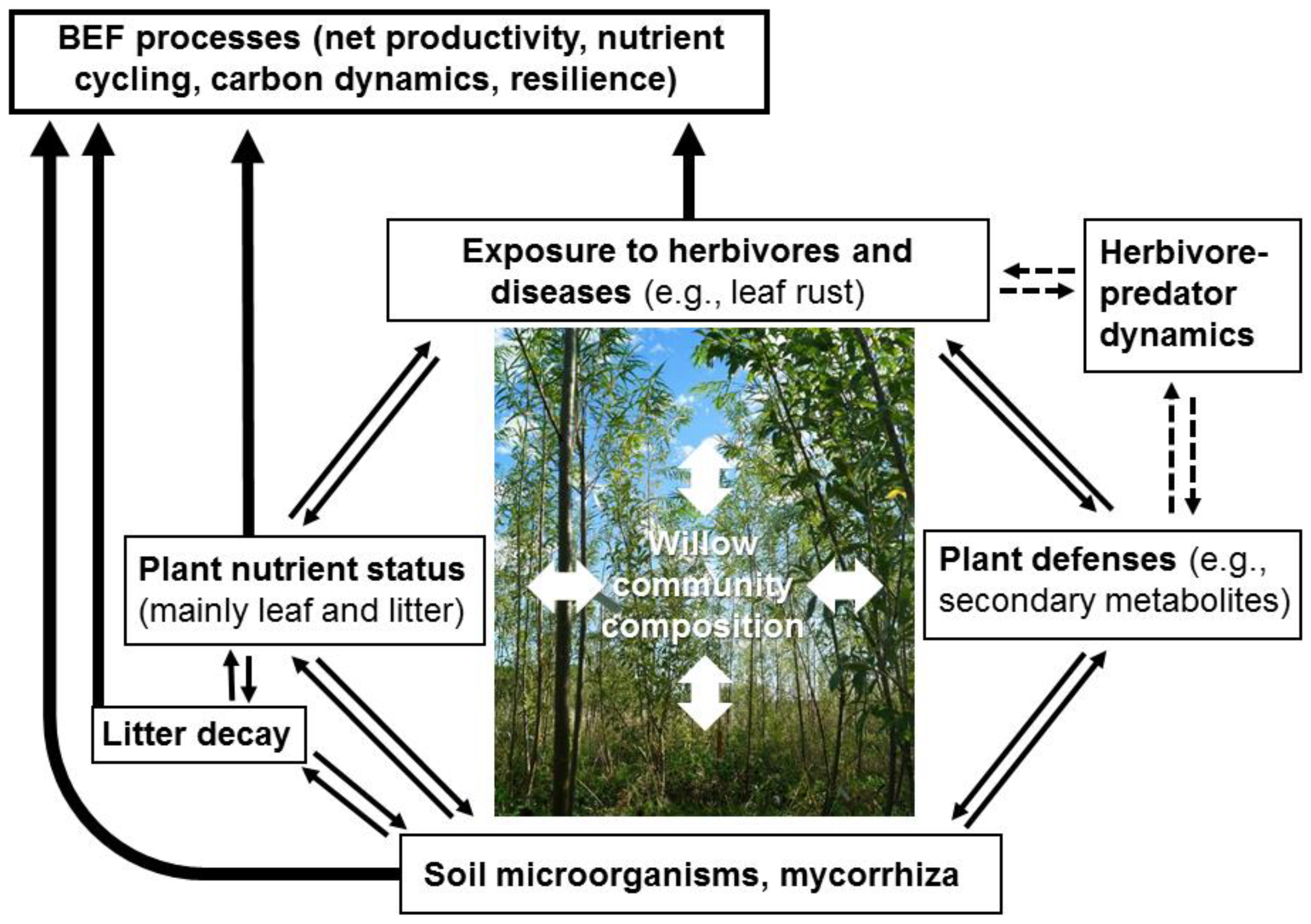
Willow Short-Rotation Coppice as Model System for Exploring Ecological Theory on Biodiversity–Ecosystem Function
Abstract
1. Introduction
2. Tree Diversity and Productivity
3. Tree Diversity and Above-Ground Trophic Interactions
4. Tree Diversity and Below-Ground Trophic Interactions
5. Discussion and Conclusions
- Willows are perennial and fast-growing.
- Willow genotypes are easily cloned, reducing a source of variation in the system.
- Willows are dual mycorrhizal (i.e., associated with arbuscular and ectomycorrhizal fungi), providing the consideration of both partly contrary impacts of the two main types of mycorrhiza formation on carbon cycling.
- Willows promote the soil faunal abundance and diversity and are appropriate model systems for the investigation of the soil food web based on their no-till management.
- Well-established field and greenhouse experimental willow model systems with varying levels of genotypic diversity are already part of professional biodiversity networks [32].
- Regular shoot harvests allow for the study of temporal patterns in shorter (i.e., within one cutting cycle of three-year) and longer time scales (i.e., across subsequent cutting cycles).
- Short-rotation willow systems have characteristics that are similar in functionality to other perennial systems such as grasslands, for which much of the relevant BEF theory development has been achieved.
- The short-rotation practice of controlled removal of above ground plant parts while below ground parts are sustained implies low risk for competitive exclusion and extinction of genotypes, and thereby enhanced opportunities for the study of the mechanisms underlying plant–plant and plant–environment (abiotic and biotic) interactions in a BEF context.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dickmann, D.I.; Kuzovkina, J. Poplars and Willows of the World, With Emphasis on Silviculturally Important Species. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; Cabi Publishing: Wallingford, UK, 2014; pp. 8–91. [Google Scholar]
- Weih, M.; Hansson, P.A.; Ohlsson, J.A.; Sandgren, M.; Schnürer, A.; Rönnberg-Wästljung, A.C. Sustainable willow production for biofuel use. In Achieving Carbon-Negative Bioenergy Systems from Plant Materials; Saffron, C., Ed.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2019; ISBN 978-1-78676-2528. in press. [Google Scholar]
- Larsson, S.; Nordh, N.E.; Farrell, J.; Tweddle, P. Manual for SRC Willow Growers; Lantmännen Agroenergi AB: Örebro, Sweden, 2007; p. 18. [Google Scholar]
- Weih, M. Evidence for increased sensitivity to nutrient and water stress in a fast-growing hybrid willow compared with a natural willow clone. Tree Physiol. 2001, 21, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Weih, M.; Nordh, N.E. Characterising willows for biomass and phytoremediation: Growth, nitrogen and water use of 14 willow clones under different irrigation and fertilisation regimes. Biomass Bioenergy 2002, 23, 397–413. [Google Scholar] [CrossRef]
- Glynn, C.; Ronnberg-Wastljung, A.; Julkunen-Tiitto, R.; Weih, M. Willow genotype, but not drought treatment, affects foliar phenolic concentrations and leaf-beetle resistance. Entomol. Exp. Appl. 2004, 113, 1–14. [Google Scholar] [CrossRef]
- Baum, C.; Toljander, Y.K.; Eckhardt, K.-U.; Weih, M. The significance of host-fungus combinations in ectomycorrhizal symbioses for the chemical quality of willow foliage. Plant Soil 2009, 323, 213–224. [Google Scholar] [CrossRef]
- Weih, M.; Nordh, N.E. Determinants of biomass production in hybrid willows and prediction of field performance from pot studies. Tree Physiol. 2005, 25, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Weih, M. Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): Effects on shoot growth and nitrogen economy. Tree Physiol. 2009, 29, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Karp, A.; Taylor, G. Defining leaf traits linked to yield in short-rotation coppice Salix. Biomass Bioenergy 2004, 26, 417–431. [Google Scholar] [CrossRef]
- Loreau, M.; Hector, A. Partitioning selection and complementarity in biodiversity experiments. Nature 2001, 412, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. In Annual Review of Ecology, Evolution, and Systematics, Volume 45; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2014; Volume 45, pp. 471–493. [Google Scholar]
- Schulze, E.D.; Bouriaud, O.; Weber, U.; Roscher, C.; Hessenmoeller, D.; Kroiher, F.; Schall, P. Management breaks the natural productivity-biodiversity relationship in forests and grassland: An opinion. For. Ecosyst. 2018, 5. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Gonzalez, A.; Byrnes, J.E.K.; Cardinale, B.J.; Duffy, J.E.; Gamfeldt, L.; Griffin, J.N.; Hooper, D.; Hungate, B.A.; Paquette, A.; et al. A general biodiversity-function relationship is mediated by trophic level. Oikos 2017, 126, 18–31. [Google Scholar] [CrossRef]
- Holzwarth, F.; Ruger, N.; Wirth, C. Taking a closer look: Disentangling effects of functional diversity on ecosystem functions with a trait-based model across hierarchy and time. R. Soc. Open Sci. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Weih, M.; Ronnberg-Wastljung, A.; Glynn, C. Genetic basis of phenotypic correlations among growth traits in hybrid willow (Salix dasyclados x S-viminalis) grown under two water regimes. New Phytol. 2006, 170, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Weih, M.; Bonosi, L.; Ghelardini, L.; Ronnberg-Wastljung, A.C. Optimizing nitrogen economy under drought: Increased leaf nitrogen is an acclimation to water stress in willow (Salix spp.). Ann. Bot. 2011, 108, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Agren, G.I.; Weih, M. Plant stoichiometry at different scales: Element concentration patterns reflect environment more than genotype. New Phytol. 2012, 194, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Fransson, P.M.A.; Toljander, Y.K.; Baum, C.; Weih, M. Host plant-ectomycorrhizal fungus combination drives resource allocation in willow: Evidence for complex species interaction from a simple experiment. Ecoscience 2013, 20, 112–121. [Google Scholar] [CrossRef]
- Puentes, A.; Torp, M.; Weih, M.; Bjorkman, C. Direct effects of elevated temperature on a tri-trophic system: Salix, leaf beetles and predatory bugs. Arthropod Plant Interact. 2015, 9, 567–575. [Google Scholar] [CrossRef]
- Hoeber, S.; Arranz, C.; Nordh, N.E.; Baum, C.; Low, M.; Nock, C.; Scherer-Lorenzen, M.; Weih, M. Genotype identity has a more important influence than genotype diversity on shoot biomass productivity in willow short-rotation coppices. Glob. Chang. Biol. Bioenergy 2018, 10, 534–547. [Google Scholar] [CrossRef]
- Schuldt, A.; Assmann, T.; Brezzi, M.; Buscot, F.; Eichenberg, D.; Gutknecht, J.; Hardtle, W.; He, J.S.; Klein, A.M.; Kuhn, P.; et al. Biodiversity across trophic levels drives multifunctionality in highly diverse forests. Nat. Commun. 2018, 9, 10. [Google Scholar] [CrossRef]
- Weih, M.; Hoeber, S.; Beyer, F.; Fransson, P. Traits to ecosystems: The ecological sustainability challenge when developing future energy crops. Front. Energy Res. 2014, 2, 1–5. [Google Scholar] [CrossRef]
- Albertsson, J.; Verwijst, T.; Hansson, D.; Bertholdsson, N.O.; Ahman, I. Effects of competition between short-rotation willow and weeds on performance of different clones and associated weed flora during the first harvest cycle. Biomass Bioenergy 2014, 70, 364–372. [Google Scholar] [CrossRef]
- Welc, M.; Lundkvist, A.; Verwijst, T. Effects of Cutting Phenology (Non-dormant Versus Dormant) on Early Growth Performance of Three Willow Clones Grown Under Different Weed Treatments and Planting Dates. Bioenergy Res. 2017, 10, 1094–1104. [Google Scholar] [CrossRef]
- Baum, S.; Weih, M.; Busch, G.; Kroiher, F.; Bolte, A. The impact of Short Rotation Coppice plantations on phytodiversity. Landbauforsch. Volkenrode 2009, 59, 163–170. [Google Scholar]
- Baum, S.; Bolte, A.; Weih, M. High value of short rotation coppice plantations for phytodiversity in rural landscapes. Glob. Chang. Biol. Bioenergy 2012, 4, 728–738. [Google Scholar] [CrossRef]
- Odum, E.P. Srategy of ecosystem development. Science 1969, 164, 262. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.D.; Kay, J.J. Life as a manifestation of the 2nd law of thermodynamics. Math. Comput. Model. 1994, 19, 25–48. [Google Scholar] [CrossRef]
- Isbell, F.; Calcagno, V.; Hector, A.; Connolly, J.; Harpole, W.S.; Reich, P.B.; Scherer-Lorenzen, M.; Schmid, B.; Tilman, D.; van Ruijven, J.; et al. High plant diversity is needed to maintain ecosystem services. Nature 2011, 477, 199–202. [Google Scholar] [CrossRef]
- Liang, J.J.; Crowther, T.W.; Picard, N.; Wiser, S.; Zhou, M.; Alberti, G.; Schulze, E.D.; McGuire, A.D.; Bozzato, F.; Pretzsch, H.; et al. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, 12. [Google Scholar] [CrossRef]
- Verheyen, K.; Vanhellemont, M.; Auge, H.; Baeten, L.; Baraloto, C.; Barsoum, N.; Bilodeau-Gauthier, S.; Bruelheide, H.; Castagneyrol, B.; Godbold, D.; et al. Contributions of a global network of tree diversity experiments to sustainable forest plantations. AMBIO 2016, 45, 29–41. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.L.; Thomson, K.T. Plant diversity and ecosystem productivity: Theoretical considerations. Proc. Natl. Acad. Sci. USA 1997, 94, 1857–1861. [Google Scholar] [CrossRef]
- Turnbull, L.A.; Levine, J.M.; Loreau, M.; Hector, A. Coexistence, niches and biodiversity effects on ecosystem functioning. Ecol. Lett. 2013, 16, 116–127. [Google Scholar] [CrossRef]
- Grossman, J.J.; Vanhellemont, M.; Barsoum, N.; Bauhus, J.; Bruelheide, H.; Castagneyrol, B.; Cavender-Bares, J.; Eisenhauer, N.; Ferlian, O.; Gravel, D.; et al. Synthesis and future research directions linking tree diversity to growth, survival, and damage in a global network of tree diversity experiments. Environ. Exp. Bot. 2018, 152, 68–89. [Google Scholar] [CrossRef]
- Hoeber, S.; Fransson, P.; Prieto-Ruiz, I.; Manzoni, S.; Weih, M. Two Salix Genotypes Differ in Productivity and Nitrogen Economy When Grown in Monoculture and Mixture. Front. Plant Sci. 2017, 8, 12. [Google Scholar] [CrossRef]
- Dillen, M.; Vanhellemont, M.; Verdonckt, P.; Maes, W.H.; Steppe, K.; Verheyen, K. Productivity, stand dynamics and the selection effect in a mixed willow clone short rotation coppice plantation. Biomass Bioenergy 2016, 87, 46–54. [Google Scholar] [CrossRef]
- Mundt, C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002, 40, 381–410. [Google Scholar] [CrossRef]
- Dawson, W.M.; McCracken, A.R. The performance of polyclonal stands in short rotation coppice willow for energy production. Biomass Bioenergy 1995, 8, 1–5. [Google Scholar] [CrossRef]
- McCracken, A.R.; Walsh, L.; Moore, P.J.; Lynch, M.; Cowan, P.; Dawson, M.; Watson, S. Yield of willow (Salix spp.) grown in short rotation coppice mixtures in a long-term trial. Ann. Appl. Biol. 2011, 159, 229–243. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Jactel, H.; Vacher, C.; Brockerhoff, E.G.; Koricheva, J. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 2014, 51, 134–141. [Google Scholar] [CrossRef]
- McCracken, A.R.; Dawson, W.M. Growing clonal mixtures of willow to reduce effect of Melampsora epitea var. epitea. Eur. J. For. Pathol. 1997, 27, 319–329. [Google Scholar] [CrossRef]
- Plath, M.; Dorn, S.; Riedel, J.; Barrios, H.; Mody, K. Associational resistance and associational susceptibility: Specialist herbivores show contrasting responses to tree stand diversification. Oecologia 2012, 169, 477–487. [Google Scholar] [CrossRef]
- Schuldt, A.; Bruelheide, H.; Hardtle, W.; Assmann, T.; Li, Y.; Ma, K.P.; von Oheimb, G.; Zhang, J.Y. Early positive effects of tree species richness on herbivory in a large-scale forest biodiversity experiment influence tree growth. J. Ecol. 2015, 103, 563–571. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Whitham, T.G.; Bailey, J.K.; Schweitzer, J.A.; Shuster, S.M.; Bangert, R.K.; LeRoy, C.J.; Lonsdorf, E.V.; Allan, G.J.; DiFazio, S.P.; Potts, B.M.; et al. A framework for community and ecosystem genetics: From genes to ecosystems. Nat. Rev. Genet. 2006, 7, 510–523. [Google Scholar] [CrossRef]
- Koricheva, J.; Hayes, D. The relative importance of plant intraspecific diversity in structuring arthropod communities: A meta-analysis. Funct. Ecol. 2018, 32, 1704–1717. [Google Scholar] [CrossRef]
- Fritz, R.S.; Price, P.W. Genetic variation among plants and insect community structure—Willows and sawflies. Ecology 1988, 69, 845–856. [Google Scholar] [CrossRef]
- Fritz, R.S. Direct and indirect effects of plant genetic variation on enemy impact. Ecol. Entomol. 1995, 20, 18–26. [Google Scholar] [CrossRef]
- Larsson, S.; Strong, D.R. Oviposition choice and larval survival of Dasineura marginemtorquens (Diptera, Cecidomyiidae) on resistant and susceptible Salix viminalis. Ecol. Entomol. 1992, 17, 227–232. [Google Scholar] [CrossRef]
- Hochwender, C.G.; Fritz, R.S. Plant genetic differences influence herbivore community structure: Evidence from a hybrid willow system. Oecologia 2004, 138, 547–557. [Google Scholar] [CrossRef]
- Dalin, P.; Kindvall, O.; Bjorkman, C. Reduced Population Control of an Insect Pest in Managed Willow Monocultures. PLoS ONE 2009, 4. [Google Scholar] [CrossRef]
- Glynn, C.; Larsson, S. Rapid gall midge adaptation to a resistant willow genotype. Agric. For. Entomol. 2000, 2, 115–121. [Google Scholar] [CrossRef]
- Glynn, C.; Herms, D.A.; Orians, C.M.; Hansen, R.C.; Larsson, S. Testing the growth-differentiation balance hypothesis: Dynamic responses of willows to nutrient availability. New Phytol. 2007, 176, 623–634. [Google Scholar] [CrossRef]
- Muller, M.; Klein, A.M.; Scherer-Lorenzen, M.; Nock, C.A.; Staab, M. Tree genetic diversity increases arthropod diversity in willow short rotation coppice. Biomass Bioenergy 2018, 108, 338–344. [Google Scholar] [CrossRef]
- Moritz, K.K.; Parachnowitsch, A.L.; Julkunen-Tiitto, R.; Bjorkman, C.; Ayres, M.P.; Stenberg, J.A. Roe deer prefer mixed-sex willow stands over monosexual stands but do not discriminate between male and female plants. Environ. Exp. Bot. 2018, 146, 62–67. [Google Scholar] [CrossRef]
- Kaplan, I.; Thaler, J.S. Do plant defenses enhance or diminish prey suppression by omnivorous Heteroptera? Biol. Control 2011, 59, 53–60. [Google Scholar] [CrossRef]
- Bjorkman, C.; Hoglund, S.; Eklund, K.; Larsson, S. Effects of leaf beetle damage on stem wood production in coppicing willow. Agric. For. Entomol. 2000, 2, 131–139. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Rasmann, S.; Castagneyrol, B.; Mooney, K.A. Plant diversity effects on insect herbivores and their natural enemies: Current thinking, recent findings, and future directions. Curr. Opin. Insect Sci. 2016, 14, 1–7. [Google Scholar] [CrossRef]
- Ronnberg-Wastljung, A.C.; Ahman, I.; Glynn, C.; Widenfalk, O. Quantitative trait loci for resistance to herbivores in willow: Field experiments with varying soils and climates. Entomol. Exp. Appl. 2006, 118, 163–174. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Vander Heijden, E.W. Differential benefits of arbuscular mycorrhizal and ectomycorrhizal infection of Salix repens. Mycorrhiza 2001, 10, 185–193. [Google Scholar] [CrossRef]
- Simard, S.W.; Durall, D.M. Mycorrhizal networks: A review of their extent, function, and importance. Can. J. Bot. Rev. Can. Bot. 2004, 82, 1140–1165. [Google Scholar] [CrossRef]
- Rooney, D.C.; Killham, K.; Bending, G.D.; Baggs, E.; Weih, M.; Hodge, A. Mycorrhizas and biomass crops: Opportunities for future sustainable development. Trends Plant Sci. 2009, 14, 542–549. [Google Scholar] [CrossRef]
- Baum, C.; Leinweber, P.; Weih, M.; Lamersdorf, N.; Dimitriou, I. Effects of short rotation coppice with willows and poplar on soil ecology. Landbauforsch. Volkenrode 2009, 59, 183–196. [Google Scholar]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 8. [Google Scholar] [CrossRef]
- Gamfeldt, L.; Snall, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Froberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 8. [Google Scholar] [CrossRef]
- Simard, S.W.; Jones, M.D.; Durall, D.M. Carbon and nutrient fluxes within and between mycorrhizal plants. Mycorrhizal Ecol. 2002, 157, 33–74. [Google Scholar]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 11. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae—A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; van der Heijden, M.G.A.; Cornelissen, J.H.C.; Makarov, M.I.; Onipchenko, V.G.; Maslov, M.N.; Akhmetzhanova, A.A.; van Bodegom, P.M. Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol. 2015, 208, 280–293. [Google Scholar] [CrossRef]
- Baum, C.; Eckhardt, K.U.; Hahn, J.; Weih, M.; Dimitriou, I.; Leinweber, P. Impact of poplar on soil organic matter quality and microbial communities in arable soils. Plant Soil Environ. 2013, 59, 95–100. [Google Scholar] [CrossRef]
- Baum, C.; Hrynkiewicz, K.; Leinweber, P.; Meissner, R. Heavy-metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix x dasyclados). J. Plant Nutr. Soil Sci. Z. Pflanzenernahr. Bodenkd. 2006, 169, 516–522. [Google Scholar] [CrossRef]
- Fillion, M.; Brisson, J.; Guidi, W.; Labrecque, M. Increasing phosphorus removal in willow and poplar vegetation filters using arbuscular mycorrhizal fungi. Ecol. Eng. 2011, 37, 199–205. [Google Scholar] [CrossRef]
- Baum, C.; Hrynkiewicz, K. Clonal and seasonal shifts in communities of saprotrophic microfungi and soil enzyme activities in the mycorrhizosphere of Salix spp. J. Plant Nutr. Soil Sci. Z. Pflanzenernahr. Bodenkd. 2006, 169, 481–487. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Baum, C.; Leinweber, P.; Weih, M.; Dimitriou, I. The significance of rotation periods for mycorrhiza formation in Short Rotation Coppice. For. Ecol. Manag. 2010, 260, 1943–1949. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Toljander, Y.K.; Baum, C.; Fransson, P.M.A.; Taylor, A.F.S.; Weih, M. Correspondence of ectomycorrhizal diversity and colonisation of willows (Salix spp.) grown in short rotation coppice on arable sites and adjacent natural stands. Mycorrhiza 2012, 22, 603–613. [Google Scholar] [CrossRef]
- Vanbeveren, S.P.P.; Ceulemans, R. Biodiversity in short-rotation coppice. Renew. Sustain. Energy Rev. 2019, 111, 34–43. [Google Scholar] [CrossRef]
- Baum, C.; Hrynkiewicz, K.; Szymanska, S.; Vitow, N.; Hoeber, S.; Fransson, P.M.A.; Weih, M. Mixture of Salix Genotypes Promotes Root Colonization with Dark Septate Endophytes and Changes P Cycling in the Mycorrhizosphere. Front. Microbiol. 2018, 9, 10. [Google Scholar] [CrossRef]
- Crotty, F.V.; Blackshaw, R.P.; Adl, S.M.; Inger, R.; Murray, P.J. Divergence of feeding channels within the soil food web determined by ecosystem type. Ecol. Evol. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Bakker, M.R.; Brunner, I.; Ashwood, F.; Bjarnadottir, B.; Bolger, T.; Børja, I.; Carnol, M.; Cudlin, P.; Dalsgaard, L.; Erktan, A.; et al. Belowground Biodiversity Relates Positively to Ecosystem Services of European Forests. Front. For. Glob. Chang. 2019, 2. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Weih, M.; Abalos Romero, M.; Charles, J.; Hurst, S.; McIvor, I.; Karp, A.; Trybush, S.; Labrecque, M.; Teodorescu, T.I. Salix: Botany and global horticulture. Hortic. Rev. 2008, 34, 447–489. [Google Scholar]
- Fabio, E.S.; Smart, L.B. Differential growth response to fertilization of ten elite shrub willow (Salix spp.) bioenergy cultivars. Trees Struct. Funct. 2018, 32, 1061–1072. [Google Scholar] [CrossRef]
- Connolly, J.; Cadotte, M.W.; Brophy, C.; Dooley, A.; Finn, J.; Kirwan, L.; Roscher, C.; Weigelt, A. Phylogenetically diverse grasslands are associated with pairwise interspecific processes that increase biomass. Ecology 2011, 92, 1385–1392. [Google Scholar] [CrossRef]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The contribution of species richness and composition to bacterial services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef]
- Bell, T.; Lilley, A.K.; Hector, A.; Schmid, B.; King, L.; Newman, J.A. A Linear Model Method for Biodiversity-Ecosystem Functioning Experiments. Am. Nat. 2009, 174, 836–849. [Google Scholar] [CrossRef]
- Connolly, J.; Bell, T.; Bolger, T.; Brophy, C.; Carnus, T.; Finn, J.A.; Kirwan, L.; Isbell, F.; Levine, J.; Luescher, A.; et al. An improved model to predict the effects of changing biodiversity levels on ecosystem function. J. Ecol. 2013, 101, 344–355. [Google Scholar] [CrossRef]
- Brophy, C.; Dooley, A.; Kirwan, L.; Finn, J.A.; McDonnell, J.; Bell, T.; Cadotte, M.W.; Connolly, J. Biodiversity and ecosystem function: Making sense of numerous species interactions in multi-species communities. Ecology 2017, 98, 1771–1778. [Google Scholar] [CrossRef]
| Trait | Minimum | Maximum | Genotypes and Growth Conditions | Source |
|---|---|---|---|---|
| Shoot biomass per plant (kg) | 0.3 | 1.5 | 6 genotypes, 4 treatments | [8] |
| Total leaf area (m2) | 0.6 | 2.5 | ||
| Root biomass fraction (-) | 0.1 | 0.2 | ||
| Specific leaf area (mm2 g−1) | 9524 | 13,089 | ||
| Leaf N concentration (%) | 1.7 | 3.3 | ||
| Bud burst date (day of year) | 76 | 115 | 6 genotypes, 4 treatments | [9] |
| Biomass yield (Mg ha−1 year−1) | 6 | 16 | 5 genotypes, 1 treatment | [10] |
| Specific leaf area (mm2 g−1) | 12,129 | 14,923 | ||
| Final individual leaf area (mm2) | 2060 | 4573 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weih, M.; Glynn, C.; Baum, C. Willow Short-Rotation Coppice as Model System for Exploring Ecological Theory on Biodiversity–Ecosystem Function. Diversity 2019, 11, 125. https://doi.org/10.3390/d11080125
Weih M, Glynn C, Baum C. Willow Short-Rotation Coppice as Model System for Exploring Ecological Theory on Biodiversity–Ecosystem Function. Diversity. 2019; 11(8):125. https://doi.org/10.3390/d11080125
Chicago/Turabian StyleWeih, Martin, Carolyn Glynn, and Christel Baum. 2019. "Willow Short-Rotation Coppice as Model System for Exploring Ecological Theory on Biodiversity–Ecosystem Function" Diversity 11, no. 8: 125. https://doi.org/10.3390/d11080125
APA StyleWeih, M., Glynn, C., & Baum, C. (2019). Willow Short-Rotation Coppice as Model System for Exploring Ecological Theory on Biodiversity–Ecosystem Function. Diversity, 11(8), 125. https://doi.org/10.3390/d11080125