New Material of Paleocene-Eocene Pellornis (Aves: Gruiformes) Clarifies the Pattern and Timing of the Extant Gruiform Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Geological Setting
2.2. Character Matrix
2.3. Body Size Estimation
2.4. Phylogenetic Analysis
3. Results
3.1. Systematic Paleontology
3.1.1. Type Species
3.1.2. Emended Differential Diagnosis
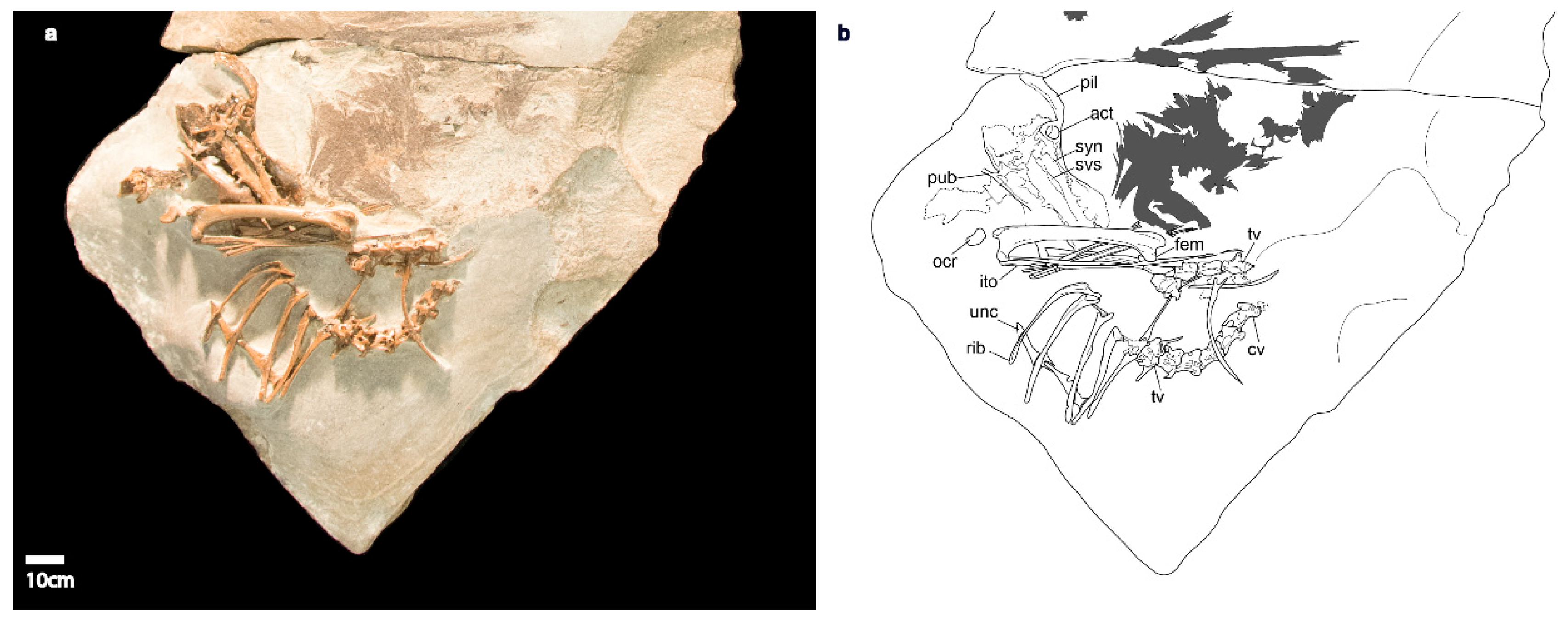
3.1.3. Holotype Specimen
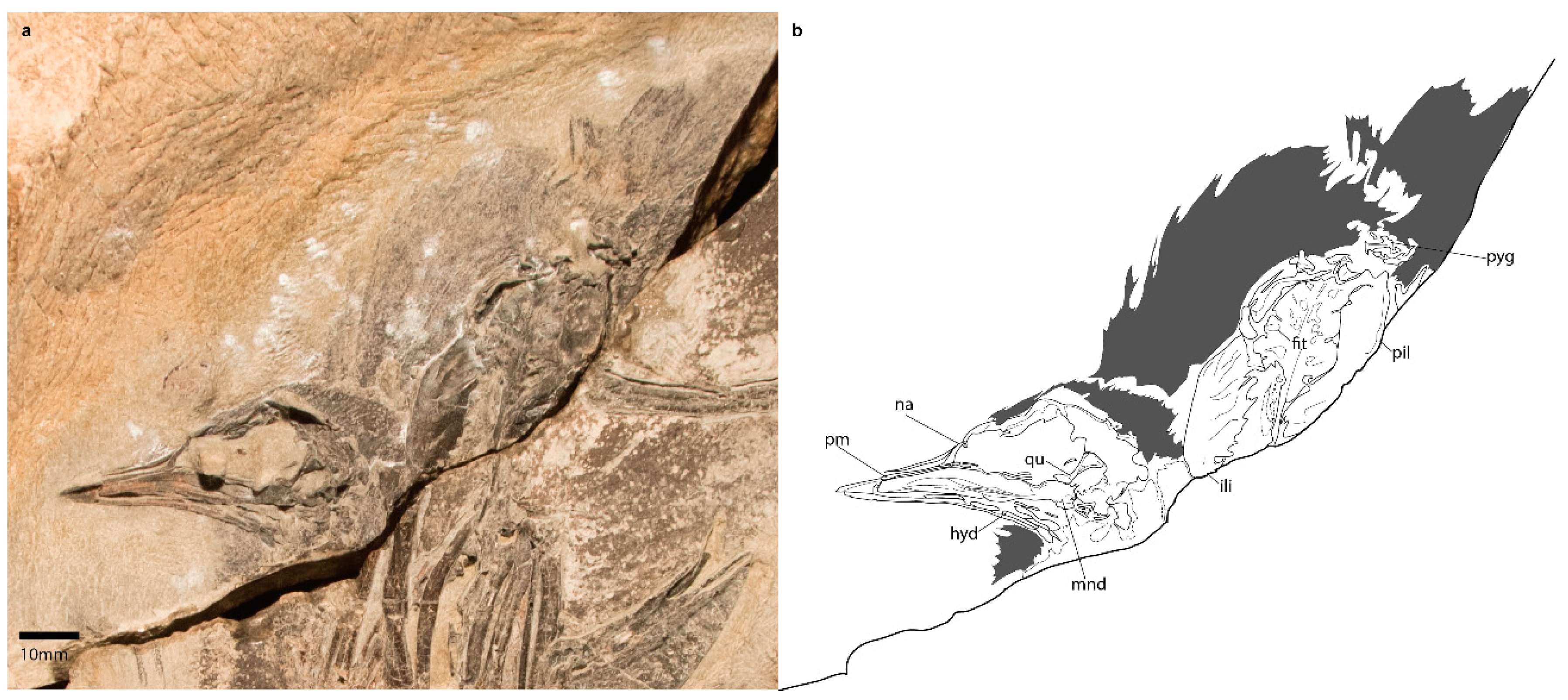
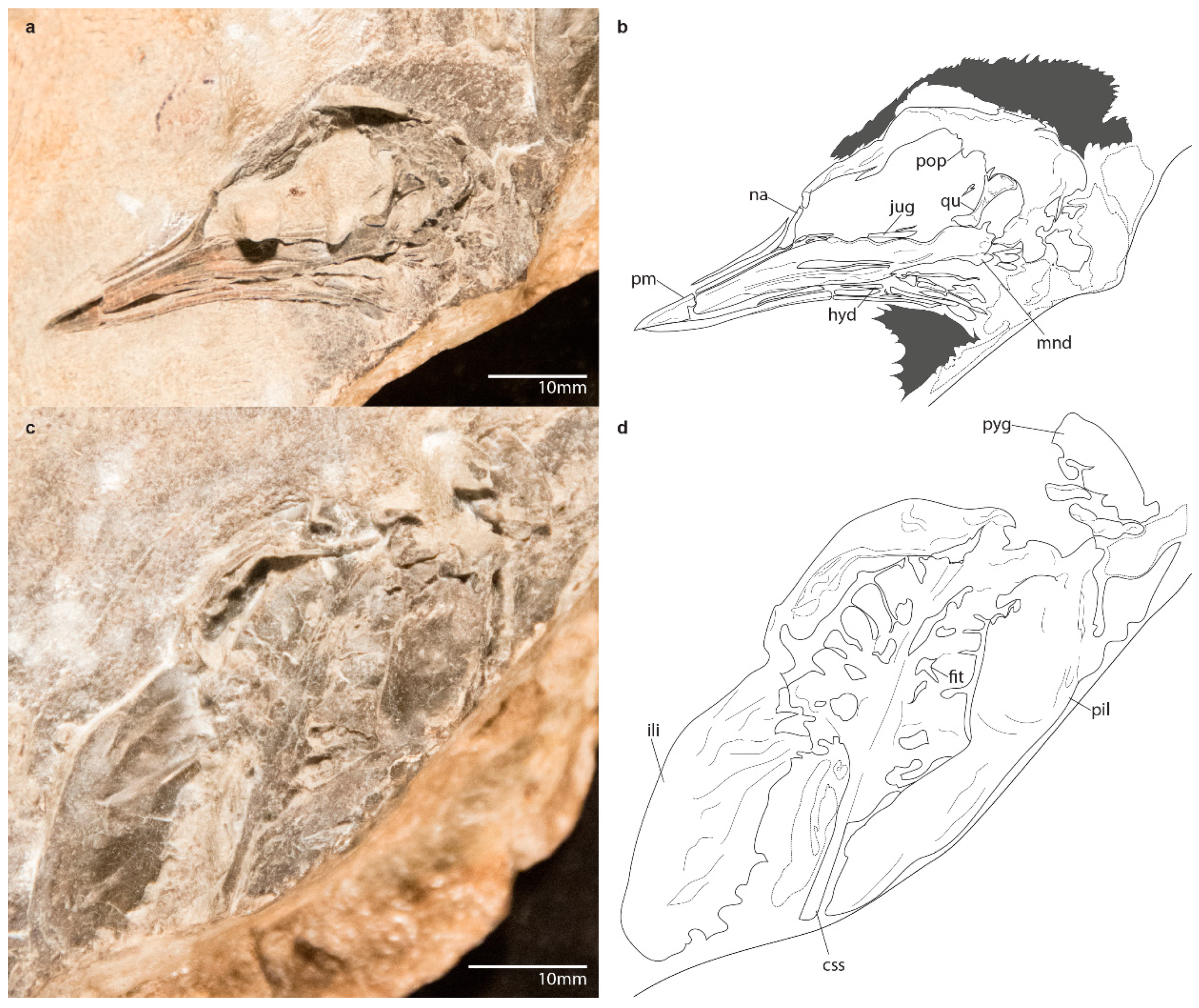
3.1.4. Referred Specimen
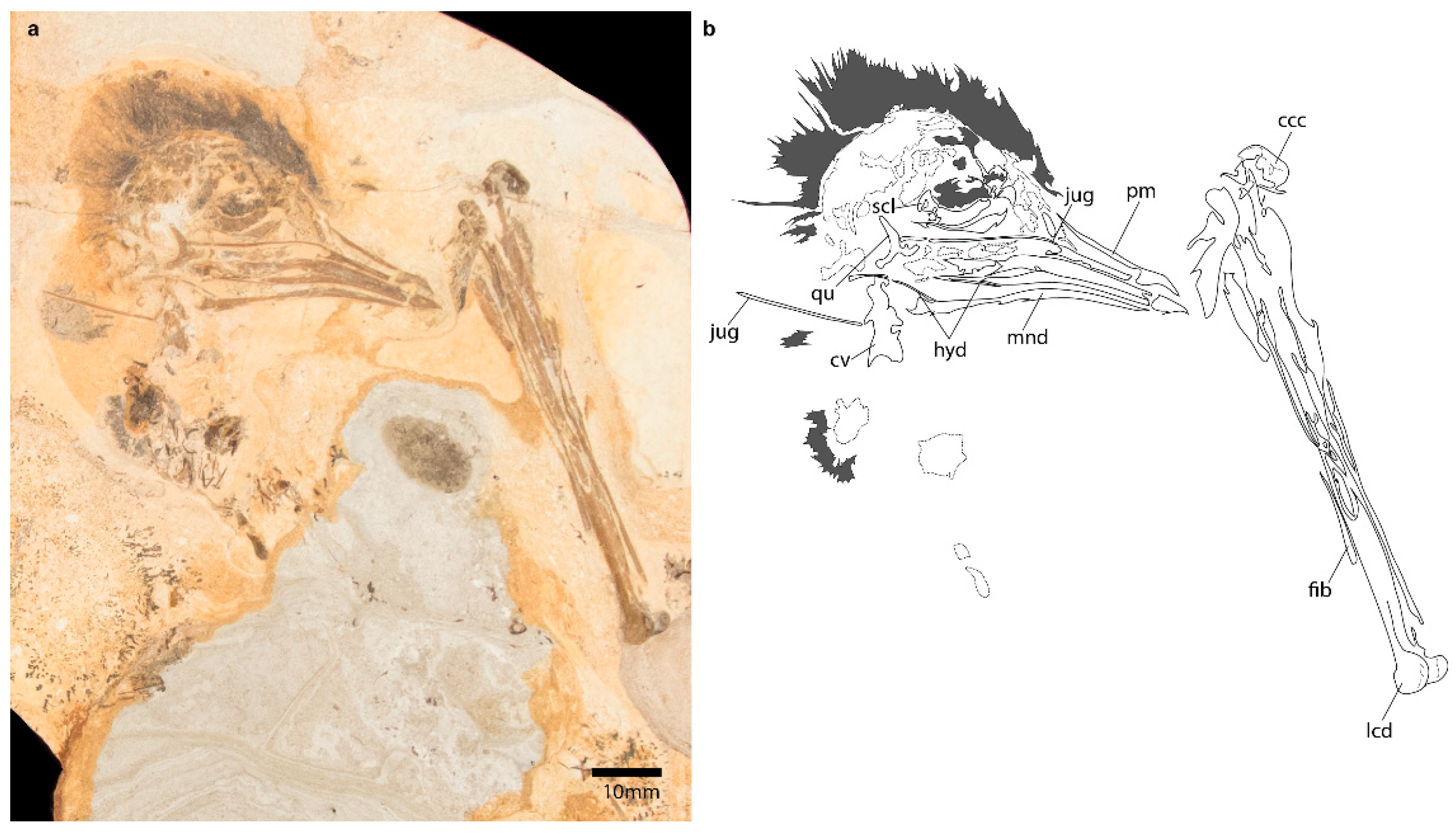
3.1.5. Referred Specimen
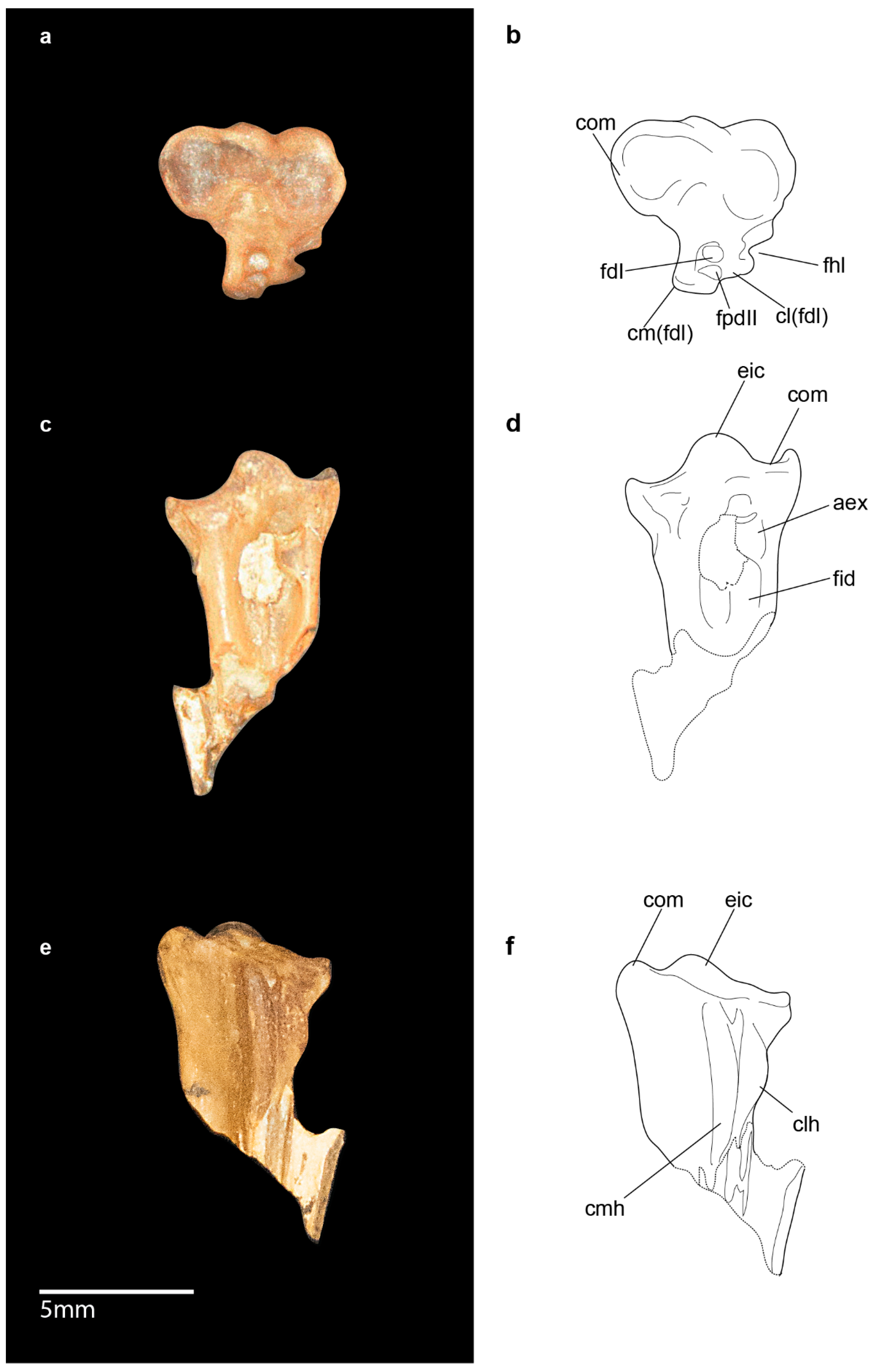
3.2. Emended Description and Remarks
3.2.1. Skull
3.2.2. Axial Skeleton
3.2.3. Pectoral Girdle
3.2.4. Forelimb
3.2.5. Pelvic Girdle
3.2.6. Hindlimb
3.2.7. Intratendinous Ossifications
3.2.8. Plumage
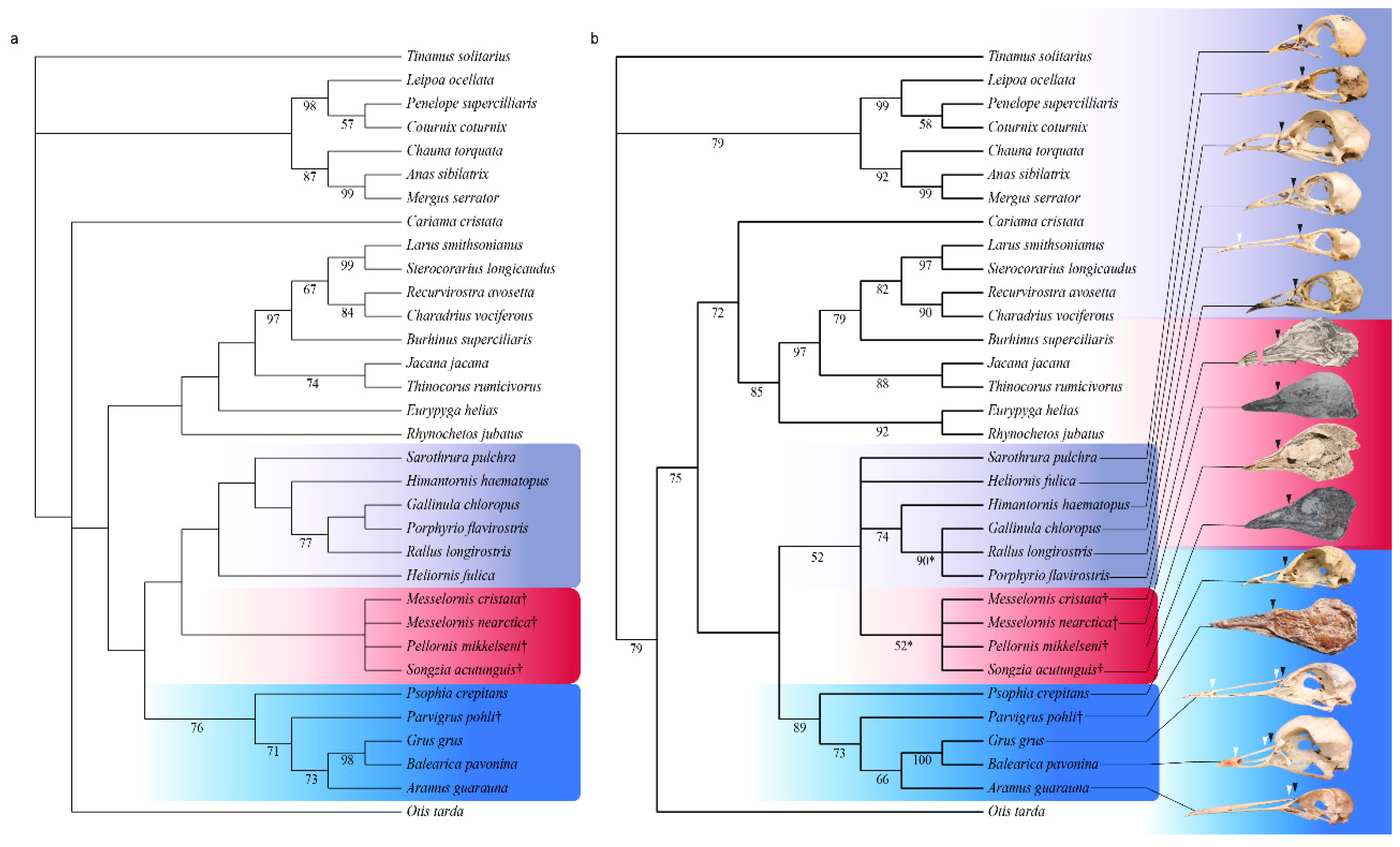
3.3. Phylogenetic Results
3.4. Body Size
4. Discussion
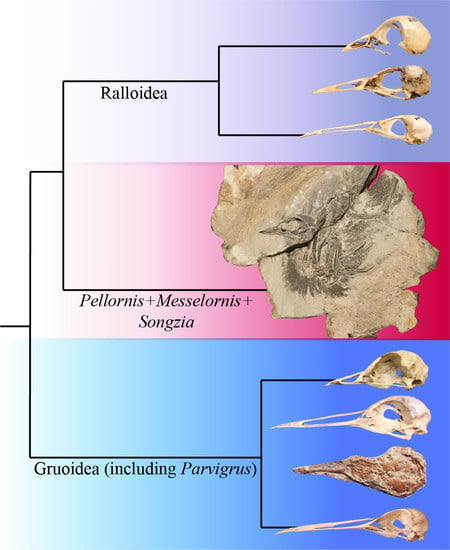
4.1. Phylogenetic Position of Pellornis, Songzia and Messelornithidae
4.2. Possible Monophyly of a Pellornis + Messelornis Clade
4.3. Divergence Times of Gruiformes
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ericson, P.G.P.; Anderson, C.L.; Britton, T.; Elzanowski, A.; Johansson, U.S.; Källersjö, M.; Ohlson, J.I.; Parsons, T.J.; Zuccon, D.; Mayr, G. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol. Lett. 2006, 2, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Prum, R.O.; Berv, J.S.; Dornburg, A.; Field, D.J.; Townsend, J.P.; Lemmon, E.M.; Lemmon, A.R. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 2015, 526, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Ksepka, D.; Phillips, M.J. Avian diversification patterns across the K-Pg boundary: Influence of calibrations, datasets, and model misspecification. Ann. Mo. Bot. Gard. 2015, 100, 300–328. [Google Scholar] [CrossRef]
- Claramunt, S.; Cracraft, J.L. A new time tree reveals Earth history’s imprint on the evolution of modern birds. Sci. Adv. 2015, 1, e1501005. [Google Scholar] [CrossRef] [PubMed]
- Berv, J.S.; Field, D.F. Genomic Signature of an Avian Lilliput Effect across the K-Pg Extinction. Syst. Biol. 2018, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Field, D.J.; Berv, J.S.; Hsiang, A.Y.; Lanfear, R.; Landis, M.J.; Dornburg, A. Timing the extant avian radiation: The rise of modern birds, and the importance of modeling molecular rate variation. PeerJ Prepr. 2019, 7. [Google Scholar] [CrossRef]
- Ericson, P.G.P. Evolution of terrestrial birds in three continents: Biogeography and parallel radiations. J. Biogeogr. 2012, 39, 813–824. [Google Scholar] [CrossRef]
- Mayr, G. Avian higher level biogeography: Southern Hemispheric origins or Southern Hemispheric relicts? J. Biogeogr. 2017, 44, 950–962. [Google Scholar] [CrossRef]
- Cracraft, J.; Claramunt, S. Conceptual and analytical worldviews shape differences about global avian biogeography. J. Biogeogr. 2017, 44, 950–962. [Google Scholar] [CrossRef]
- Field, D.J.; Hsiang, A.Y. A North American stem turaco, and the complex biogeographic history of modern birds. BMC Evolut. Biol. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Musser, G.M.; Cracraft, J. A new morphological dataset reveals a novel relationship for the adzebills of New Zealand (Aptornis) and provides a foundation for total evidence neoavian phylogenetics. Am. Mus. Novit. 2019, 3927, 1–70. [Google Scholar] [CrossRef]
- Houde, P.; Cooper, A.; Leslie, E.; Shand, A.E.; Montano, G.A. Phylogeny and evolution of 12S rDNA in gruiformes (Aves). In Avian Molecular Evolution and Systematics; Mindell, D.P., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 117–154. [Google Scholar]
- Livezey, B.C. A phylogenetic analysis of the Gruiformes (Aves) based on morphological characters, with an emphasis on the rails (Rallidae). Philos. Trans. R. Soc. Lond. B 1998, 353, 2077–2151. [Google Scholar] [CrossRef]
- Fain, M.G.; Krajewski, C.; Houde, P. Phylogeny of “core Gruiformes” (Aves: Grues) and resolution of the Limpkin-Sungrebe problem. Mol. Phylogenet. Evolut. 2007, 43, 515–529. [Google Scholar] [CrossRef] [PubMed]
- García, R.J.C.; Gibb, G.C.; Trewick, S.A. Eocene Diversificaiton of Crown Group Rails (Aves: Gruiformes: Rallidae). PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- García, R.J.C.; Gibb, G.C.; Trewick, S.A. Deep global evolutionary radiation in birds: Diversification and trait evolution in the cosmopolitan bird family Rallidae. Mol. Phylogenet. Evolut. 2014, 81, 96–108. [Google Scholar] [CrossRef]
- Boast, A.P.; Chapman, B.; Herrera, M.B.; Worthy, T.H.; Scofield, R.P.; Tennyson, A.J.D.; Houde, P.; Bunce, M.; Cooper, A.; Mitchell, K.J. Mitochondrial genomes from New Zealand’s extinct Adzebills (Aves: Aptornithidae: Aptornis) support a sister-taxon relationship with the Afro-Madagascan Sarothruridae. Diversity 2019, 11, 24. [Google Scholar] [CrossRef]
- Mayr, G. Paleogene Fossil Birds; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mayr, G. Avian Evolution: The Fossil Record of Birds and Its Paleobiological Significance; John Wiley and Sons Ltd.: West Sussex, UK, 2017. [Google Scholar]
- Mayr, G.; Smith, T. New Paleocene bird fossils from the North Sea Basin in Belgium and France. Geol. Belg. 2019, 22, 35–46. [Google Scholar] [CrossRef]
- Olson, S.L. A Synopsis of the Fossil Rallidae Rails of the World: A Monograph of the Family Rallidae; Codine: Boston, MA, USA, 1977. [Google Scholar]
- Cracraft, J. Systematics and evolution of the Gruiformes (class Aves): 3. Phylogeny of the suborder Grues. Bull. Am. Mus. Nat. Hist. 1973, 151, 1. [Google Scholar]
- Hesse, A. A new species of Messelornis (Aves: Gruiformes: Messelornithidae) from the Middle Eocene Green River Formation. In Contributions in Science: Papers in Avian Paleontotogy Honoring Pierce Brodkorb; Campbell, K.E., Ed.; Los Angeles County Museum of Natural History: Los Angeles, CA, USA, 1992; Number 330; pp. 171–178. [Google Scholar]
- Hesse, A. Die Messelornithidae-eine neue Familie der Kranichartigen (Aves: Gruiformes: Rhynocheti) aus dem Tertiär Europas und Nordamerikas. J. Ornithol. 1988, 129, 83–95. [Google Scholar] [CrossRef]
- Mayr, G. The early Eocene birds of the Messel fossil site: A 48 million-year-old bird community adds a temporal perspective to the evolution of tropical avifaunas. Biol. Rev. 2017, 92, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Grande, L. The Lost World of Fossil Lake: Snapshots from Deep Time; The University of Chicago Press: Chicago, IL, USA, 2013. [Google Scholar]
- Weidig, I. New birds from the Lower Eocene Green River Formation, North America. In Proceedings of the VII International Meeting of the Society of Avian Paleontology and Evolution, Sydney, Australia, 18–23 August 2008 ; Boles, W.E., Worthy, T.H., Eds.; Records of the Australian Museum, Australian Museum: Sydney, Australia, 2010; pp. 29–44. [Google Scholar]
- Mourer-Chauviré, C. The Messelornithidae (Aves: Gruiformes) from the Paleogene of France. Cour. Forsch. Senckenberg 1995, 181, 95–105. [Google Scholar]
- Hesse, A. Die Beschreibung der Messelornithidae (Aves: Gruiformes: Rhynocheti) aus dem Alttertiär Europas und Nordamerikas. Cour. Forsch. Senckenberg 1990, 128, 1–176. [Google Scholar]
- Peters, D.S. Zoogeographical relationships of the Eocene avifauna from Messel (Germany). In Proceedings of the Acta XX Congressus Internationalis Ornithologici, Christchurch, New Zealand, 2–9 December 1990; Bell, B.D., Cossee, R.O., Flux, J.E.C., Heather, B.D., Hitchmough, R.A., Robertson, C.J.R., Williams, M.J., Eds.; New Zealand Ornithological Congress Trust Board: Christchurch, New Zealand, 1991; pp. 572–577. [Google Scholar]
- Feduccia, A. The Origin and Evolution of Birds; Yale University Press: New Haven, CA, USA, 1996. [Google Scholar]
- Cracraft, J. Avian evolution, Gondwana biogeography and the Cretaceous-Tertiary mass extinction event. Proc. R. Soc. Lond. B 2001, 268, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Phylogenetic relationships of the early Tertiary Messel rails (Aves, Messelornithidae). Senckenbergiana Lethaea 2004, 84, 317–322. [Google Scholar] [CrossRef]
- Bertelli, S.; Chiappe, L.M.; Mayr, G. A new Messel rail from the early Eocene fur Formation of Denmark (Aves, Messelornithidae). J. Syst. Paleontol. 2011, 9, 551–562. [Google Scholar] [CrossRef]
- Wang, M.; Mayr, G.; Zhang, J.; Zhou, Z. Two new skeletons of the enigmatic, rail-like avian taxon Songzia Hou, 1990 (Songziidae) from the early Eocene of China. Alcheringa Aust. J. Paleontol. 2012, 36, 487–499. [Google Scholar] [CrossRef]
- Hou, L.H. An Eocene bird from Songzi, Hubei province. Vertebr. Palasiat. 1990, 28, 34–42. [Google Scholar]
- Mayr, G.; Hervet, S.; Buffetaut, E. On the diverse and widely ignored Paleocene avifauna of Menat (Puy-de-Dôme, France): New taxonomic records and unusual soft tissue preservation. Geol. Mag. 2019, 156, 572–584. [Google Scholar] [CrossRef]
- Hoch, E. Notes on palaeornithology and on a New Bird from the Early Tertiary North Sea Region. Geological Society of Denmark, Online Series, 1. 1997. Available online: http://www.2dgf.dk/online/bird.htm (accessed on 15 September 2017).
- Kristoffersen, A.V. The Avian Diversity in the Latest Paleocene-Earliest Eocene Fur Formation, Denmark. Unpublished. Ph.D. Thesis, Geological Institute, University of Copenhagen, København, Denmark, 2002. [Google Scholar]
- Chambers, L.M.; Pringle, M.; Fitton, G.; Larsen, L.M.; Pedersen, A.K.; Parrish, R. Recalibration of the Palaeocene-Eocene boundary (P-E) using high precision U-Pb and Ar-Ar isotopic dating: Abstracts. In Proceedings of the EGS-AGU-EUG Joint Assembly, Nice, France, 6–11 April 2003; pp. 9681–9682. [Google Scholar]
- Schultz, B.P. (Muserum, Division of Natural History, Havnevej, Skive, Denmark). Personal communication, 2019.
- Baumel, J.J.; Witmer, L.M. Osteologia. In Handbook of Avian Anatomy: Nomina Anatomica Avium; Baumel, J.J., King, A.S., Breazile, J.E., Evans, H.E., Vanden Berge, J.C., Eds.; Publications of the Nuttall Ornithological Club: Cambridge, MA, USA, 1993; pp. 45–132. [Google Scholar]
- Mayr, G.; Clarke, J.A. The deep divergences of neornithine birds: A phylogenetic analysis of morphological characters. Cladistics 2003, 19, 527–553. [Google Scholar] [CrossRef]
- Worthy, T.H.; Boles, W.E. Australlus, a New Genus for Gallinula disneyi (Aves: Rallidae) and a Description of a New Species from Oligo-Miocene Deposits at Riversleigh, Northwestern Queensland, Australia. Rec. Aust. Mus. 2011, 63, 61–77. [Google Scholar] [CrossRef]
- Mayr, G. A chicken-sized crane precursor from the early Oligocene of France. Naturwissenschaften 2005, 92, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G. Parvigruidae (Aves, core Gruiformes) from the early Oligocene of Belgium. Paleodivers. Paleoenviron. 2013, 93, 77–89. [Google Scholar] [CrossRef]
- Olson, S. A classification of the Rallidae. Wilson Bull. 1973, 65, 381–416. [Google Scholar]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.L.; Harshman, J.; et al. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Kaufman, S.G. MorphoBank 3.0: Web Application for Morphological Phylogenetics and Taxonomy. 2012. Available online: https://morphobank.org/index.php/FAQ/Index (accessed on 15 September 2017).
- Field, D.J.; Lynner, C.; Brown, C.; Darroch, S.A.F. Skeletal Correlates for Body Mass Estimation in Modern and Fossil Flying Birds. PLoS ONE 2013, 8, e82000. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0a164 (X86); Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Photographic Facsimile; British Museum of Natural History: London, UK, 1758. [Google Scholar]
- Pycraft, W.P. On the morphology and phylogeny of the Palaeognathae (Ratitae and Crypturi) and Neognathae (Carinatae). Trans. Zool. Soc. Lond. 1900, 15, 149–290. [Google Scholar] [CrossRef]
- Vinther, J.; Briggs, D.E.G.; Prum, R.O.; Saranathan, V. The colour of fossil feathers. Biol. Lett. 2008, 4. [Google Scholar] [CrossRef]
- Mayr, G. A rail (Aves, Rallidae) from the early Oligocene of Germany. ARDEA 2006, 94, 23–31. [Google Scholar]
- Ksepka, D.T. Broken gears in the avian molecular clock: New phylogenetic analyses support stem galliform status for Gallinuloides wyomingensis and rallid affinities for Amitabha urbsinterdictensis. Cladistics 2009, 25, 173–197. [Google Scholar] [CrossRef]
- Colleary, C.; Dolocan, A.; Gardner, J.; Singh, S.; Wuttke, M.; Rabenstein, R.; Habersetzer, J.; Schaal, S.; Feseha, M.; Clemens, M.; et al. Chemical, experimental, and morphological evidence for diagenetically altered melanin in exceptionally preserved fossils. Proc. Natl. Acad. Sci. USA 2015, 112, 12592–12597. [Google Scholar] [CrossRef] [PubMed]
- Zusi, R.L. A functional and evolutionary analysis of rhynchokinesis in birds. Smithson. Contrib. Zool. 1984, 395, 1–40. [Google Scholar] [CrossRef]
- Dunning, J.B. Handbook of Avian Body Masses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Feduccia, A. Avian extinction at the end of the Cretaceous: Assessing the magnitude and subsequent explosive radiation. Cretac. Res. 2014, 50, 1–15. [Google Scholar] [CrossRef]
- Suh, A. The phylogenomic forest of bird trees contains a hard polytomy at the root of Neoaves. Zool. Scr. 2016, 45, 50–62. [Google Scholar] [CrossRef]
- Fain, M.G.; Houde, P. Parallel radiations in the primary clades of birds. Evolution 2004, 58, 2558–2573. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Kimball, R.T.; Pandey, A.; Hosner, P.A.; Braun, M.J.; Hackett, S.J.; Han, K.L.; Harshman, J.; Huddleston, C.J.; Kingston, S.; et al. Why do phylogenomic data sets yield conflicting trees? Data type influences the avian tree of life more than taxon sampling. Syst. Biol. 2017, 66, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Field, D.J.; Bercovici, A.; Berv, J.S.; Regan, D.; Fastovsky, D.E.; Lyson, T.R.; Vajda, V.; Gauthier, J.A. Early evolution of modern birds structured by global forest collapse at the end-Cretaceous mass extinction. Curr. Biol. 2018, 28, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G.; Smith, R. Ducks, rails and limicoline waders (Aves: Ansriformes, Gruiformes, Charadriiformes) form the lowermost Oligocene of Belgium. Geobios 2001, 334, 547–561. [Google Scholar] [CrossRef]
- De Pietri, V.L.; Mayr, G. Reappraisal of early Miocene rails (Aves, Rallidae) from central France: Diversity and character evolution. J. Zool. Syst. Evolut. Res. 2014, 52, 312–322. [Google Scholar] [CrossRef]
- Livezey, B.C.; Zusi, R.L. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy: I. methods and characters. Bull. Carnegie Mus. Nat. Hist. 2006, 37, 1–95. [Google Scholar] [CrossRef]
- Mayr, G. The phylogenetic relationships of the early Tertiary Primoscenidae and Sylphornithidae and the sister taxon of crown group piciform birds. J. Ornithol. 2004, 145, 188–198. [Google Scholar] [CrossRef]
- Bertelli, S.; Lindowm, B.E.K.; Dyke, G.J.; Mayr, G. Another charadriiform-like bird from the Lower Eocene of Denmark. Paleontol. J. 2013, 11, 1282–1301. [Google Scholar] [CrossRef]
| Taxon | Prum et al. [3] (nuclear) | Claramunt and Cracraft [5] (nuclear) | García R et al. [16] (mtDNA) | García R et al. [17] (mtDNA) | Boast et al. [18] (mtDNA) |
|---|---|---|---|---|---|
| Gruiformes | mid-Eocene 39 (29–61) Ma | Paleocene-Eocene boundary 57 (62–52.0) Ma | K-Pg boundary 67 (75–59) Ma | K-Pg boundary 65 (74–57) Ma | K-Pg boundary 65.4 (52.0–80.4) Ma |
| Gruoidea | early Oligocene 32 (52–15) Ma | mid-Eocene 42 (55-25) Ma | late Eocene 35 (52–23) Ma | late Eocene 35 (46–26) Ma | Paleocene 59.1(43.6–76.9) Ma |
| Ralloidea | mid-Oligocene 29 (46–16) Ma | mid-Eocene 43 (54-32) Ma | early Eocene 52 (60–44) Ma | mid-Paleocene 60 (70–52) Ma | early Eocene 54.7 (41.4–70.0) Ma |
| Rallidae | early Miocene 16 (26–4) Ma | mid-Eocene 43 (54-32) Ma | mid-Eocene 41 (48–33) Ma | late Eocene 38 (45–32) Ma | late Eocene 40.5 (28.4–53.9) Ma |
| Element | MGUH 29278 | DK664 | FUM 1681a |
|---|---|---|---|
| Skull (total length) | 50.3 | 48.5 | |
| Synsacrum (total length) | 45.7 | ||
| Cranial margin of synsacrum to caudal margin of pubis | |||
| Femur (total length) | 41.1 | 39.1 | |
| Femur (midshaft width) | 3.0 | ||
| Tibiotarsus (total length) | 69.1 |
| Group Name | Species Sampled and Specimen Numbers |
|---|---|
| Charadriiformes | Larus smithsonianus (YPM 102675) |
| Gruoidea | Psophia crepitans (AMNH 29322, YPM 102505, USNM 320971, USNM 429974), Aramus guarauna (AMNH 24194, YPM 102505, USNM 612025, USNM 226809), Balearica regulorum (YPM 105863, AMNH 10699) |
| Ralloidea | Heliornis fulica (USNM 345807, USNM 623068, USNM 19159, USNM 321493, YPM 109145), Sarothrura pulchra (AMNH 4235, USNM 291778, USNM 292395), Rallus longirostris (M-10359, USNM 501940, USNM 525876), Himantornis haematopus (AMNH 4183, USNM 318581), Gallinula chloropus (AMNH 28451, YPM 103042, USNM 499259), Fulica cornuta (AMNH 10207, M-11460), Porphyrio flavirostris (YPM 109144), Amaurornis flavirostra (YPM 103778), Aramides ypecaha (YPM 101029, USNM 614591, USNM 635722, USNM 614593) |
| Eurypygidae | Eurypyga helias (AMNH 3750, AMNH 4293, USNM 623251) |
| Rhynochetidae | Rhynochetos jubatus (AMNH 1326, AMNH 554, USNM 612087) |
| Extinct Taxa | Pellornis mikkelseni (MGUH 29278, DK664, FUM 1681a), Songzia acutunguis [36], Messelornis nearctica [24,28], Itardiornis hessae [29], Australlus disneyi and A. gagensisi [45], Parvigrus pohli [46,47] |
| Group Name | Unconstrained (Tree 1) | Prum et al. [3] Constraint |
|---|---|---|
| Gruiformes (including extinct taxa) | 5(1), 10(1) 1, 30(1), 45(1), 46(0), 70(0), 81(1), 98(1), 99(1) | 10(1) 1, 30(1), 45(1), 46(0), 51(1), 96(0), 98(0), 99(1) |
| Gruoidea (including P. pohli) | 4(0), 33(2), 39(1), 48(1) *, 49(1), 51(1) *, 56(1) 1, 59(2) *, 77(1), 82(0), 86(1) 1, 87(1) * | 33(2), 34(1), 39(1), 48(1) *, 49(1), 54(0) *, 56(1) 1, 59(2) *, 77(1), 86(1) 1, 87(1) * |
| P. pohli + (Aramidae + Gruidae) | 1(1) *, 19(1), 22(1), 31(1), 45(0), 53(1), 65(0), 70(1), 72(1) 1, 84(0) | 1(1) *, 19(1), 22(1), 31(1), 45(0), 53(1), 65(0), 70(1), 72(1) 1, 84(0) |
| Aramidae + Gruidae | 69(1) *, 91(1) * | 69(1) *, 91(1) * |
| Gruidae | 12(0) *, 32(0) *, 42(1) 1*, 55(2) *, 84(1) *, 92(0) *, 96(1) * | 12(0) *, 32(0) *, 42(1) 1*, 55(2) *, 84(1) *, 92(0) *, 96(1) * |
| Pellornis + Messelornis + Songzia + Ralloidea | 34(0), 41(1), 62(0), 67(1), 80(1) 1, 88(1), 91(1), 92(0), 93(1) 1 | 41(1), 62(0), 67(1), 80(1) 1, 88(1), 91(1), 92(0), 93(1) 1 |
| Ralloidea | 2(0), 79(0) *, 90(1) * | 2(0), 57(1) *, 79(0) *, 90(1) * |
| Sarothruridae + Rallidae | 51(1) *, 57(1) *, 82(0), 101(1) | - |
| Rallidae | 4(0) *, 54(1) *, 77(1) *, 91(0) | 77(1) *, 91(0) |
| R. longisrostris + (G. chloropus +P. flavirostris) | 23(0) *, 38(1) *, 97(1) *, 101(0) * | 23(0) *, 38(1) *, 97(1) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musser, G.; Ksepka, D.T.; Field, D.J. New Material of Paleocene-Eocene Pellornis (Aves: Gruiformes) Clarifies the Pattern and Timing of the Extant Gruiform Radiation. Diversity 2019, 11, 102. https://doi.org/10.3390/d11070102
Musser G, Ksepka DT, Field DJ. New Material of Paleocene-Eocene Pellornis (Aves: Gruiformes) Clarifies the Pattern and Timing of the Extant Gruiform Radiation. Diversity. 2019; 11(7):102. https://doi.org/10.3390/d11070102
Chicago/Turabian StyleMusser, Grace, Daniel T. Ksepka, and Daniel J. Field. 2019. "New Material of Paleocene-Eocene Pellornis (Aves: Gruiformes) Clarifies the Pattern and Timing of the Extant Gruiform Radiation" Diversity 11, no. 7: 102. https://doi.org/10.3390/d11070102
APA StyleMusser, G., Ksepka, D. T., & Field, D. J. (2019). New Material of Paleocene-Eocene Pellornis (Aves: Gruiformes) Clarifies the Pattern and Timing of the Extant Gruiform Radiation. Diversity, 11(7), 102. https://doi.org/10.3390/d11070102