Mitigating Tropical Forest Fragmentation with Natural and Semi-Artificial Canopy Bridges
Abstract
1. Introduction
2. Materials and Methods
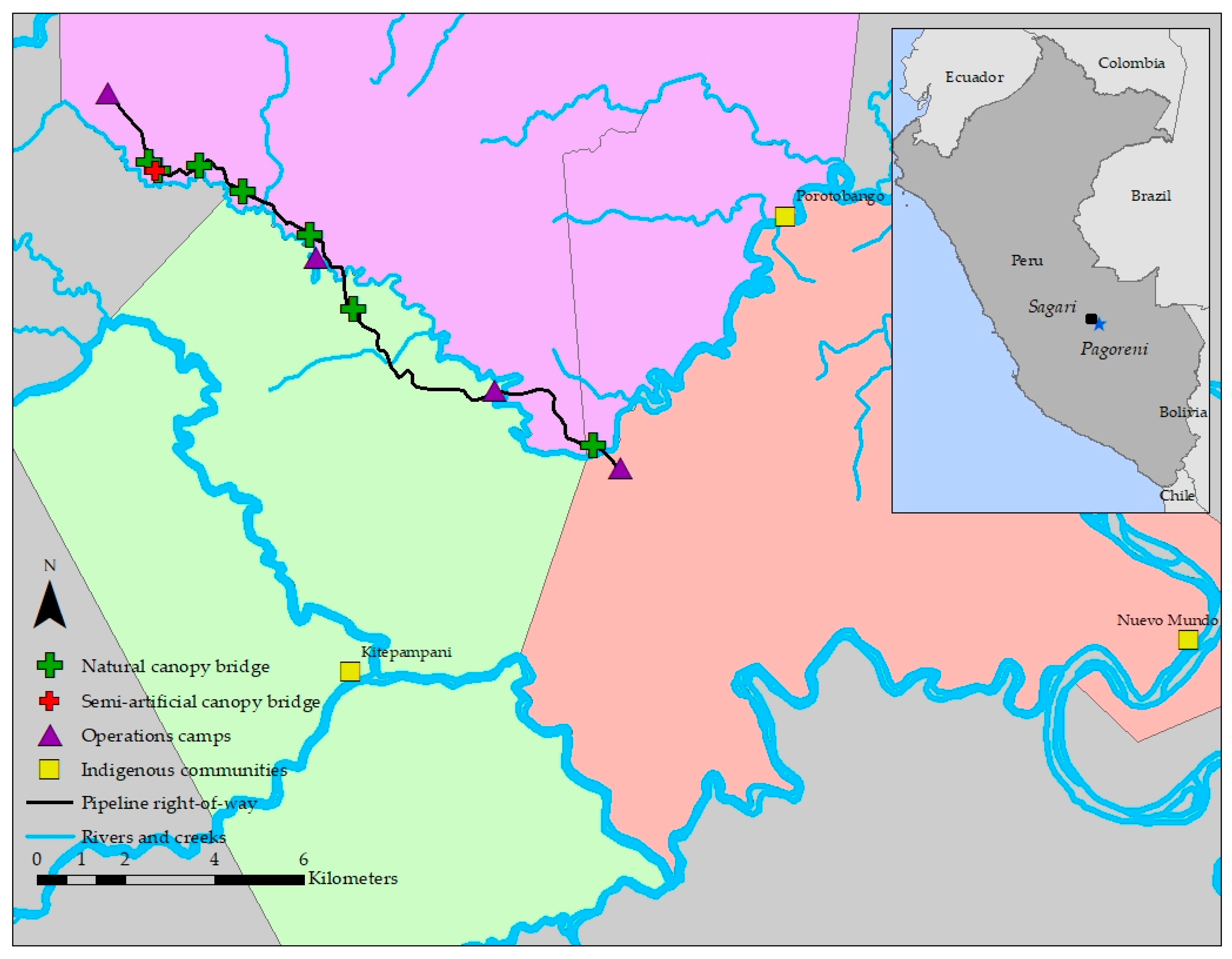
2.1. Study Site
2.2. Bridge Selection
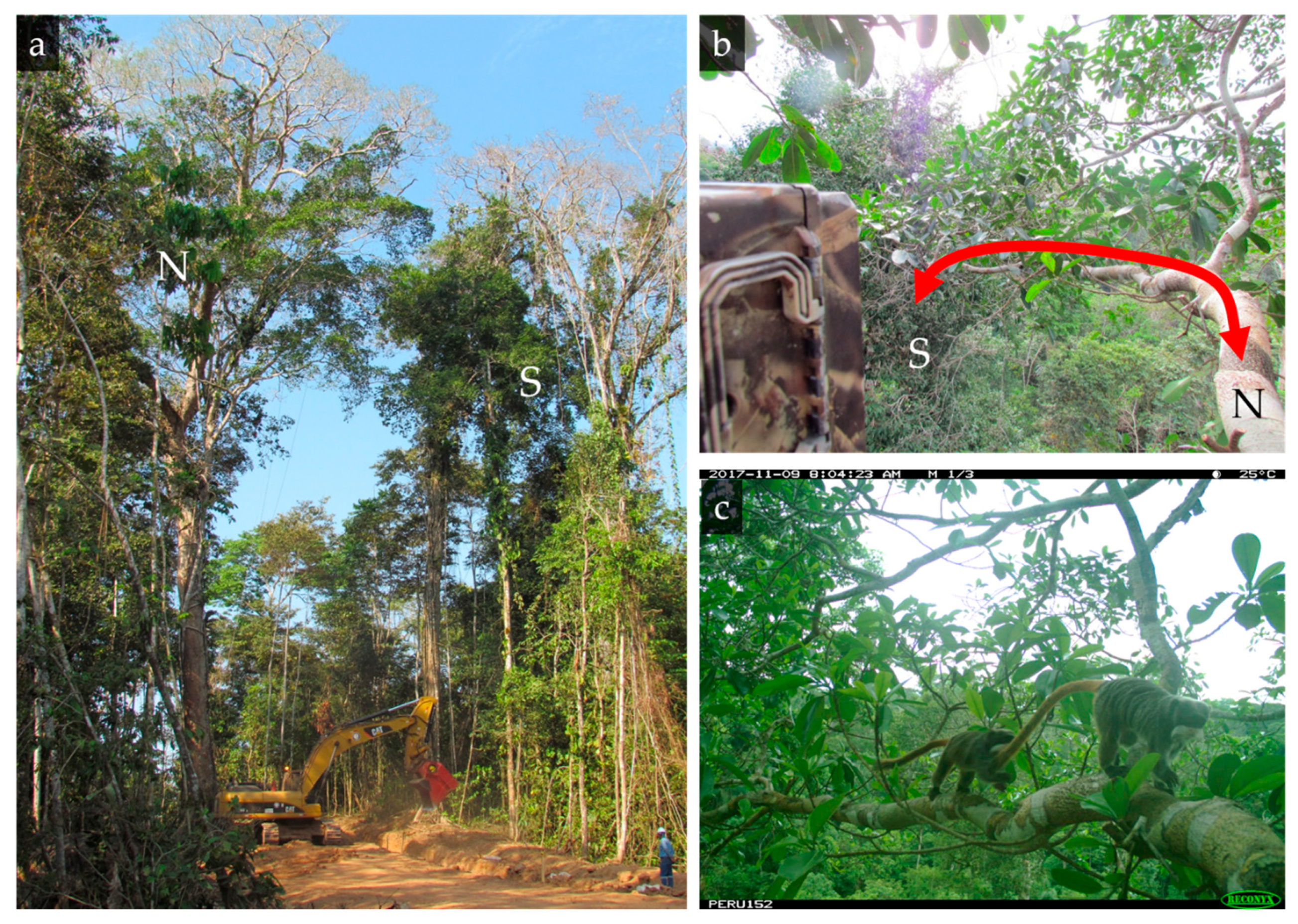
2.3. Semi-Artificial Canopy Bridge
2.4. Canopy Bridge Monitoring
3. Results
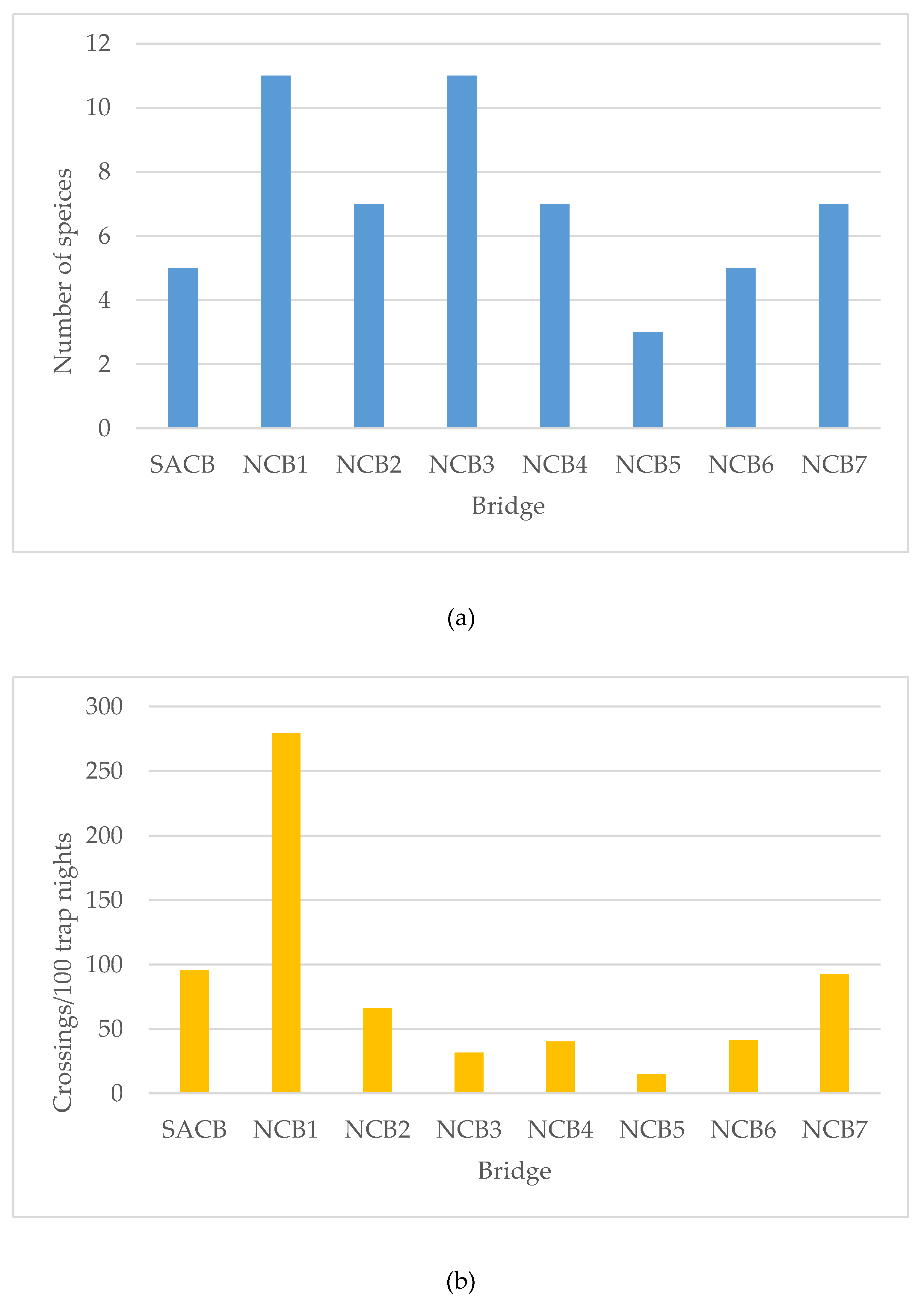
3.1. Canopy Bridge Monitoring
3.2. Semi-Artificial Canopy Bridge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.J.C.; Asner, G.P.; Knapp, D.E.; Almeyda, A.; Galvan-Gildemeister, R.; Keene, S.; Raybin, R.F.; Smith, R.C. Land-Use Allocation Protects the Peruvian Amazon. Science 2007, 317, 1233–1236. [Google Scholar] [CrossRef]
- Clements, G.R.; Lynam, A.J.; Gaveau, D.; Yap, W.L.; Lhota, S.; Goosem, M.; Laurance, S.; Laurance, W.F. Where and How Are Roads Endangering Mammals in Southeast Asia’s Forests? PLoS ONE 2014, 9, e115376. [Google Scholar] [CrossRef]
- Santos, A.M.; Tabarelli, M. Distance from roads and cities as a predictor of habitat loss and fragmentation in the caatinga vegetation of Brazil. Braz. J. Biol. 2002, 62, 897–905. [Google Scholar] [CrossRef][Green Version]
- Reed, R.A.; Johnson-Barnard, J.; Baker, W.L. Contribution of Roads to Forest Fragmentation in the Rocky Mountains. Conserv. Biol. 1996, 10, 1098–1106. [Google Scholar] [CrossRef]
- Swenson, J.J.; Carter, C.E.; Domec, J.-C.; Delgado, C.I. Gold mining in the Peruvian Amazon: global prices, deforestation, and mercury imports. PLoS ONE 2011, 6, e18875. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F. Conservation and the Global Infrastructure Tsunami: Disclose, Debate, Delay! Trends Ecol. Evol. 2018, 33, 568–571. [Google Scholar] [CrossRef]
- Alamgir, M.; Campbell, M.J.; Sloan, S.; Suhardiman, A.; Supriatna, J.; Laurance, W.F. High-risk infrastructure projects pose imminent threats to forests in Indonesian Borneo. Sci. Rep. 2019, 9, 140. [Google Scholar] [CrossRef]
- Laurance, W.F.; Peletier-Jellema, A.; Geenen, B.; Koster, H.; Verweij, P.; Van Dijck, P.; Lovejoy, T.E.; Schleicher, J.; Van Kuijk, M. Reducing the global environmental impacts of rapid infrastructure expansion. Curr. Biol. 2015, 25, R259–R262. [Google Scholar] [CrossRef]
- Gregory, T.; Carrasco-Rueda, F.; Alonso, A.; Kolowski, J.; Deichmann, J.L. Natural canopy bridges effectively mitigate tropical forest fragmentation for arboreal mammals. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Soanes, K.; Taylor, A.C.; Sunnucks, P.; Vesk, P.A.; Cesarini, S.; van der Ree, R. Evaluating the success of wildlife crossing structures using genetic approaches and an experimental design: lessons from a gliding mammal. J. Appl. Ecol. 2018, 55, 129–138. [Google Scholar] [CrossRef]
- Teixeira, F.Z.; Printes, R.C.; Fagundes, J.C.G.; Alonso, A.C.; Kindel, A. Canopy bridges as road overpasses for wildlife in urban fragmented landscapes. Biota Neotrop. 2013, 13, 117–123. [Google Scholar] [CrossRef]
- Lokschin, L.X.; Rodrigo, C.P.; Hallal Cabral, J.N.; Buss, G. Power Lines and Howler Monkey Conservation in Porto Alegre, Rio Grande do Sul, Brazil. Neotrop. Primates 2007, 14, 76–80. [Google Scholar] [CrossRef]
- Donaldson, A.; Cunneyworth, P. Case Study: Canopy Bridges for Primate Conservation. In Handbook of Road Ecology; Grilo, C., van der Ree, R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 341–343. ISBN 978-1-118-56817-0. [Google Scholar]
- Williams, B. ARCO’s Villano project: Improvised solutions in Ecuador’s rainforest. Oil Gas J. 1999, 97, 19–25. [Google Scholar]
- Thurber, M.W.; Ayarza, P.W. Canopy Bridges along a Rainforest Pipeline in Ecuador. In Proceedings of the SPE Asia Pacific Health, Safety and Environment Conference and Exhibition, Kuala Lumpur, Malaysia, 19–21 September 2005; Society of Petroleum Engineers: Kuala Lumpur, Malaysia, 2005. [Google Scholar]
- Thurber, M.W.; Abad, G.H. Rainforest Connectivity Strategies for Oil and Gas Development; Society of Petroleum Engineers: Stavanger, Norway, 2016. [Google Scholar]
- Valladares-Padua, C.; Cullen Jr, L.; Padua, S. A pole bridge to avoid primate road kills. Neotrop. Primates 1995, 3, 13–15. [Google Scholar]
- Das, J.; Biswas, J.; Bhattacherjee, P.C.; Rao, S.S. Canopy Bridges: An Effective Conservation Tactic for Supporting Gibbon Populations in Forest Fragments. In The Gibbons; Whittaker, D., Lappan, S., Eds.; Springer: New York, NY, USA, 2009; pp. 467–475. ISBN 978-0-387-88603-9. [Google Scholar]
- Lindshield, S.M. Protecting nonhuman primates in peri-urban environments: A case study of Neotropical monkeys, corridor ecology, and coastal economy in the Caribe Sur of Costa Rica. In Ethnoprimatology; Waller, M.T., Ed.; Springer: Zug, Switzerland, 2016; pp. 351–369. [Google Scholar]
- Narváez Rivera, G.; Lindshield, S.M. An experimental evaluation of crossing structures for New World monkeys in a Costa Rican wildlife sanctuary. In Proceedings of the International Primatological Society and the American Society of Primatologists, Chicago, IL, USA, 21–27 August 2016. [Google Scholar]
- Goldingay, R.L.; Rohweder, D.; Taylor, B.D. Will arboreal mammals use rope-bridges across a highway in eastern Australia? Aust. Mammal. 2013, 35, 30. [Google Scholar] [CrossRef]
- Goosem, M.; Weston, N.; Bushnell, S. Effectiveness of rope bridge arboreal overpasses and faunal underpasses in providing connectivity for rainforest fauna. In Proceedings of the 2005 International Conference on Ecology and Transportation (ICOET 2005), San Diego, CA, USA, 29 August–5 September 2005. [Google Scholar]
- Weston, N.; Goosem, M.; Marsh, H.; Cohen, M.; Wilson, R. Using canopy bridges to link habitat for arboreal mammals: successful trials in the Wet Tropics of Queensland. Aust. Mammal. 2011, 33, 93. [Google Scholar] [CrossRef]
- Walsh Perú Monitoreo Biólógico Plataforma y Flowline Sagari; Repsol Exploración Perú: Lima, Peru, 2017; p. 12.
- Alonso, A.; Dallmeier, F.; Campbell, P. Urubamba: The Biodiversity of a Peruvian Rainforest; Smithsonian Institution: Washington, DC, USA, 2001; ISBN 978-1-893912-10-6. [Google Scholar]
- Gregory, T.; Carrasco Rueda, F.; Deichmann, J.; Kolowski, J.; Costa Faura, M.; Dallmeier, F.; Alonso, A. Methods To Establish Canopy Bridges To Increase Natural Connectivity in Linear Infrastructure Development.; Society of Petroleum Engineers: Lima, Peru, 2013. [Google Scholar]
- Gregory, T.; Carrasco Rueda, F.; Deichmann, J.; Kolowski, J.; Alonso, A. Arboreal camera trapping: taking a proven method to new heights. Methods Ecol. Evol. 2014, 5, 443–451. [Google Scholar] [CrossRef]
- Walsh Perú S.A. Estudio de Impacto Ambiental para el Proyecto de desarrollo del campo Sagari—Lote 57; Repsol Exploración Perú: Lima, Perú, 2016. [Google Scholar]
- Walsh Perú S.A. Machiguenga a Reserve for Everyone; Repsol Exploración Perú: Lima, Perú, 2016. [Google Scholar]
- Walsh Perú S.A. Estudio sobre recursos naturales y medio ambiente en la Reserva Comunal Machiguenga; Perú LNG: Lima, Perú, 2007. [Google Scholar]
- Morales, V. Evaluación ecológica rápida en las Reservas Comunales Ashaninka y Machiguenga; Repsol Exploración Perú: San Isidro, Peru, 2008. [Google Scholar]
- Superina, M.; Miranda, F.R.; Abba, A.M. The 2010 anteater red list assessment. Edentata 2010, 11, 96–115. [Google Scholar] [CrossRef][Green Version]
- White, I.C.; Hughes, S.A. Trial of a bridge for reconnecting fragmented arboreal habitat for hazel dormouse Muscardinus avellanarius at Briddlesford Nature Reserve, Isle of Wight, UK. Conserv. Evid. 2019, 16, 6–11. [Google Scholar]
- Smith, D.J.; Van Der Ree, R.; Rosell, C. Wildlife crossing structures: an effective strategy to restore or maintain wildlife connectivity across roads. In Handbook of Road Ecology; Grilo, C., van der Ree, R., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 172–183. [Google Scholar]
- Soanes, K.; Vesk, P.A.; van der Ree, R. Monitoring the use of road-crossing structures by arboreal marsupials: insights gained from motion-triggered cameras and passive integrated transponder (PIT) tags. Wildl. Res. 2015, 42, 241–256. [Google Scholar] [CrossRef]
- van der Ree, R.; van der Grift, E.; Gulle, N.; Holland, K.; Mata, C.; Suarez, F. Overcoming the barrier effect of roads—How effective are mitigation strategies? An international review of the use and effectiveness of underpasses and overpasses designed to increase the permeability of roads for wildlife. In Proceedings of the 2007 International Conference on Ecology and Transportation, Raleigh, CA, USA, 20–25 May 2007; pp. 423–431. [Google Scholar]
- Yokochi, K.; Bencini, R. A remarkably quick habituation and high use of a rope bridge by an endangered marsupial, the western ringtail possum. Nat. Conserv. 2015, 11, 79–94. [Google Scholar] [CrossRef]
- Mass, V.; Rakotomanga, B.; Rakotondratsimba, G.; Razafindramisa, S.; Andrianaivomahefa, P.; Dickinson, S.; Berner, P.O.; Cooke, A. Lemur bridges provide crossing structures over roads within a forested mining concession near Moramanga, Toamasina Province, Madagascar. Conserv. Evid. 2011, 8, 11–18. [Google Scholar]
- Soanes, K.; Lobo, M.C.; Vesk, P.A.; McCarthy, M.A.; Moore, J.L.; van der Ree, R. Movement re-established but not restored: Inferring the effectiveness of road-crossing mitigation for a gliding mammal by monitoring use. Biol. Conserv. 2013, 159, 434–441. [Google Scholar] [CrossRef]
- Bissonette, J.A.; Adair, W. Restoring habitat permeability to roaded landscapes with isometrically-scaled wildlife crossings. Biol. Conserv. 2008, 141, 482–488. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]

| Family | Species | Events (rate/100 trap nights) | ||||
|---|---|---|---|---|---|---|
| Scientific Name | Common Name | Cross | Unknown | |||
| Nocturnal Events | Diurnal Events | Nocturnal Events | Diurnal Events | |||
| Aotidae | Aotus nigriceps | Black-headed night monkey | 660 (15.03) | 264 (6.01) | ||
| Atelidae | Alouatta seniculus | Red howler monkey | 14 (0.32) | 17 (0.39) | ||
| Cebidae | Cebus albifrons | White-fronted capuchin | 1 (0.02) | |||
| Cebidae | Saguinus imperator | Emperor tamarin | 50 (1.14) | 22 (0.50) | ||
| Cebidae | Sapajus macrocephalus | Brown capuchin | 11 (0.25) | 195 (4.44) | 7 (0.16) | |
| Cyclopedidae | Cyclopes didactylus | Silky anteater | 2 (0.05) | 3 (0.07) | ||
| Didelphidae | Caluromys lanatus | Brown-eared woolly opossum | 409 (9.32) | 54 (1.23) | ||
| Didelphidae | Didelphis marsupialis | Common opossum | 6 (0.14) | 7 (0.16) | ||
| Didelphidae | Marmosa sp. | Mouse opossum species | 24 (0.55) | 3 (0.07) | ||
| Echimyidae | Mesomys sp. | Spiny tree rat species | 3 (0.07) | 2 (0.05) | ||
| Erethizontidae | Coendou bicolor | Bicolored-spined porcupine | 9 (0.21) | |||
| Erethizontidae | Coendou ichillus | Streaked dwarf porcupine | 91 (2.07) | 17 (0.39) | ||
| Procyonidae | Potos flavus | Kinkajou | 706 (16.08) | 194 (4.42) | ||
| Procyonidae | Bassaricyon alleni | Eastern lowland olingo | 337 (7.68) | 46 (1.05) | ||
| Sciuridae | Hadrosciurus spadiceus | Southern Amazon red squirrel | 2 (0.05) | 5 (0.11) | 1 (0.02) | |
| Sciuridae | Notosciurus pucheranii | Andean squirrel | 4 (0.09) | |||
| Unknown mammal | 4 (0.09) | 410 (9.34) | 8 (0.18) | |||
| Total events (overall rates) | 2264 (51.57) | 269 (6.13) | 1000 (22.78) | 55 (1.25) | ||
| Total events (overall rates) | 2533 (57.70) | 1055 (24.03) | ||||
| Family | Species | Events (rate/100 trap nights) | ||
|---|---|---|---|---|
| Scientific Name | Common Name | Cross | Unknown | |
| Aotidae | Aotus nigriceps | Black-headed night monkey | 36 (17.73) | 6 (2.96) |
| Cebidae | Sapajus macrocephalus | Brown capuchin | 2 (0.99) | |
| Didelphidae | Caluromys lanatus | Brown-eared woolly opossum | 2 (0.99) | |
| Echimyidae | Mesomys sp. | Spiny tree rat species | 29 (14.29) | 21 (10.34) |
| Procyonidae | Bassaricyon alleni | Eastern lowland olingo | 95 (46.80) | 14 (6.90) |
| Procyonidae | Potos flavus | Kinkajou | 28 (13.79) | 4 (1.97) |
| Unknown mammal | 4 (1.97) | 9 (4.43) | ||
| Total events (overall rates) | 194 (95.57) | 56 (27.59) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbuena, D.; Alonso, A.; Panta, M.; Garcia, A.; Gregory, T. Mitigating Tropical Forest Fragmentation with Natural and Semi-Artificial Canopy Bridges. Diversity 2019, 11, 66. https://doi.org/10.3390/d11040066
Balbuena D, Alonso A, Panta M, Garcia A, Gregory T. Mitigating Tropical Forest Fragmentation with Natural and Semi-Artificial Canopy Bridges. Diversity. 2019; 11(4):66. https://doi.org/10.3390/d11040066
Chicago/Turabian StyleBalbuena, Diego, Alfonso Alonso, Margot Panta, Alan Garcia, and Tremaine Gregory. 2019. "Mitigating Tropical Forest Fragmentation with Natural and Semi-Artificial Canopy Bridges" Diversity 11, no. 4: 66. https://doi.org/10.3390/d11040066
APA StyleBalbuena, D., Alonso, A., Panta, M., Garcia, A., & Gregory, T. (2019). Mitigating Tropical Forest Fragmentation with Natural and Semi-Artificial Canopy Bridges. Diversity, 11(4), 66. https://doi.org/10.3390/d11040066





