First Report of the Coral-Killing Sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A New Threat to Kimberley Coral Reefs?
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Bryan, P.G. Growth rate, toxicity and distribution of the encrusting sponge Terpios sp. (Hadromerida: Suberitidae) in Guam, Mariana Islands. Micronesica 1973, 9, 237–242. [Google Scholar]
- Tang, S.L.; Hong, M.J.; Liao, M.-H.; Jane, W.N.; Chiang, P.W.; Chen, C.B.; Chen, C.A. Bacteria associated with an encrusting sponge (Terpios hoshinota) and the corals partially covered by the sponge. Environ. Microbiol. 2011, 13, 1179–1191. [Google Scholar] [CrossRef]
- Reimer, J.D.; Nozawa, Y.; Hirose, E. Domination and Disappearance of the Black Sponge: A Quarter Century after the Initial Terpios Outbreak in Southern Japan. Zool. Stud. 2011, 50, 394. [Google Scholar]
- Lin, W.J.; Soong, K.Y. Growth and prevention experiments of Terpios hoshinota surrounding Green Island. J. Natl. Park 2009, 19, 46–57. [Google Scholar]
- Thinesh, T.; Meenatchi, R.; Pasiyappazham, R.; Jose, P.A.; Selvan, M.; Kiran, G.S.; Selvin, J. Short-term in situ shading effectively mitigates linear progression of coral-killing sponge Terpios hoshinota. PLoS ONE 2017, 12, e0182365. [Google Scholar] [CrossRef]
- Rützler, K.; Muzik, K. Terpios hoshinota, a new cyanobacteriosponge threatening Pacific reefs. Sci. Mar. 1993, 57, 395–403. [Google Scholar]
- Liao, M.H.; Tang, S.L.; Hsu, C.M.; Wen, K.C.; Wu, H.; Chen, W.M.; Wang, J.T.; Meng, P.J.; Twan, W.H.; Lu, C.K.; et al. The ‘Black Disease’ of Reef-Building Corals at Green Island, Taiwan—Outbreak of a Cyanobacteriosponge, Terpios hoshinota (Suberitidae; Hadromerida). Zool. Stud. 2007, 46, 520. [Google Scholar]
- Reimer, J.D.; Mizuyama, M.; Nakano, M.; Fujii, T.; Hirose, E. Current status of the distribution of the coral-encrusting cyanobacteriosponge Terpios hoshinota in southern Japan. Galaxea 2011, 13, 35–44. [Google Scholar] [CrossRef]
- Fujii, T.; Keshavmurthy, S.; Zhou, W.; Hirose, E.; Chen, C.A.; Reimer, J.D. Coral-killing cyanobacteriosponge (Terpios hoshinota) on the Great Barrier Reef. Coral Reefs 2011, 30, 483. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, G.H.; Yan, H.Q.; Zhang, H.L. Black Disease (Terpios hoshinota): A Probable Cause for the Rapid Coral Mortality at the Northern Reef of Yongxing Island in the South China Sea. AMBIO 2012, 41, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ekins, M.; Willis, B.; Bridge, T.; Srinivasan, M.; Rowley, S.; Hooper, J. The coral killing sponge Terpios hoshinota in Kimbe Bay, Papua New Guinea. Mem. Queensl. Mus. Nat. 2017, 60, 174–175. [Google Scholar]
- de Voogd, N.; Cleary, D.F.R.; Dekker, F. The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 2013, 32, 755. [Google Scholar] [CrossRef]
- van der Ent, E.; Hoeksema, B.W.; de Voogd, N.J. Abundance and genetic variation of the coral-killing cyanobacteriosponge Terpios hoshinota in the Spermonde Archipelago, SW Sulawesi, Indonesia. J. Mar. Biol. Assoc. UK 2015, 96, 453–463. [Google Scholar] [CrossRef]
- Madduppa, H.; Schupp, P.J.; Faisal, M.R.; Sastria, M.Y.; Thoms, C. Persistent outbreaks of the ‘black disease’ sponge Terpios hoshinota in Indonesian coral reefs. Mar. Biodivers. 2015, 47, 14–15. [Google Scholar] [CrossRef]
- Tri Utami, R.; Zamani, N.P.; Madduppa, H.H. Molecular identification, abundance and distribution of the coral-killing sponge Terpios hoshinota in Bengkulu and Seribu Islands, Indonesia. Biodiversitas 2018, 19, 2238–2246. [Google Scholar]
- Montano, S.; Chou, W.H.; Chen, C.A.; Galli, P.; Reimer, J.D. First record of the coral-killing sponge Terpios hoshinota in the Maldives and Indian Ocean. Bull. Mar. Sci. 2014, 91, 97–98. [Google Scholar] [CrossRef]
- Elliott, J.; Patterson, M.; Summers, N.; Miternique, C.; Montocchio, E.; Vitry, E. How does the proliferation of the coral-killing sponge Terpios hoshinota affect benthic community structure on coral reefs? Coral Reefs 2016, 35, 1083–1095. [Google Scholar] [CrossRef]
- Plucer Rosario, G. The effect of substratum on the growth of Terpios, an encrusting sponge which kills corals. Coral Reefs 1987, 5, 197–200. [Google Scholar] [CrossRef]
- Richards, Z.; Bryce, M.; Bryce, C. The composition and structure of shallow benthic reef communities in the Kimberley, NW Australia. Rec. West Aust. Mus. Suppl. 2018, 85, 75–103. [Google Scholar] [CrossRef]
- Wilson, B. Kimberley marine biota. History and environment. Rec. West Aust. Mus. Suppl. 2014, 84, 1–18. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Michelli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.J. Reefs and shoals in Australia—Indonesian relations: Traditional Indonesian fishermen. In Australia in Asia: Episodes; Milner, A., Quilty, M., Eds.; Oxford University Press: Melbourne, Australia, 1998; pp. 111–139. [Google Scholar]
- Moloney, B.W.; Newman, S.J.; Joll, L.; Lenanton, R.C.J.; Wise, B. Are Western Australian waters the least productive waters for finfish across two oceans? A review with a focus on finfish resources in the Kimberley region and north coast subregion. J. R. Soc. West. Aust. 2011, 94, 323–332. [Google Scholar]
- Moore, C.H.; Radford, B.; Possingham, H.; Heyward, A.; Stewart, R.; Watts, M.; Prescott, J.; Newman, S.; Harvey, E.; Fisher, R.; et al. Improving spatial prioritisation for remote marine regions: Optimising biodiversity conservation and sustainable development trade-offs. Sci. Rep. 2016, 6, 32029. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Gilmour, J.P.; Heyward, A.J. Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs 2008, 27, 197–205. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Smith, L.D.; Heyward, A.J.; Baird, A.H.; Pratchett, M.S. Recovery of an isolated coral reef system following severe disturbance. Science 2013, 340, 66–71. [Google Scholar] [CrossRef]
- Ceccarelli, D.M.; Richards, Z.T.; Pratchett, M.S.; Cvitanovic, C. Rapid increase in coral cover on an isolated coral reef, the Ashmore Reef National Nature Reserve, north-western Australia. Mar. Freshw. Res. 2011, 62, 1214–1220. [Google Scholar] [CrossRef]
- Heyward, A.; Jones, R.; Travers, M.; Burns, K.; Suosaari, G.; Colquhoun, J.; Case, M.; Radford, B.; Meekan, M.; Markey, K.; et al. Montara: 2011 Shallow Reef Surveys at Ashmore, Cartier and Seringapatam Reefs; Final report for PTTEP Australasia (Ashmore Cartier) Pty. Ltd; Australian Institute of Marine Science: Townsville, Australia, 2011. [Google Scholar]
- Le Nohaic, M.; Ross, C.L.; Cornwall, C.E.; Comeau, S.; Lowe, R.; McCulloch, M.T.; Schoepf, V. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 2017, 7, 14999. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Cook, K.L.; Ryan, N.M.; Puotinen, M.L.; Green, R.H.; Shedrawi, G.; Hobbs, J.-P.A.; Thomson, D.P.; Babock, R.C.; Buckee, J.; et al. The state of Western Australia’s coral reefs. Coral Reefs 2019, 8, 1–17. [Google Scholar] [CrossRef]
- Richards, Z.; Garcia, R.; Moore, G.; Fromont, J.; Kirkendale, L.; Bryce, M.; Bryce, C.; Hara, A.; Ritchie, J.; Gomez, O.; et al. A tropical Australian refuge for photosymbiotic benthic fauna. Coral Reefs 2019, 38, 1–8. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Vargas, S.; Kelly, M.; Schnabel, K.; Mills, S.; Bowden, D.; Wörheide, G. Diversity in a Cold Hot-Spot: DNA-Barcoding Reveals Patterns of Evolution among Antarctic Demosponges (Class Demospongiae, Phylum Porifera). PLoS ONE 2015, 10, e0127573. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour. 2013, 1–13. [Google Scholar] [CrossRef]
- Meyer, C.; Geller, J.; Paulay, G. Fine scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution 2005, 59, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Redmond, N.E.; Morrow, C.C.; Thacker, R.W.; Diaz, M.C.; Boury-Esnault, N.; Cárdenas, P.; Hajdu, E.; Lôbo-Hajdu, G.; Picton, B.E.; Pomponi, S.A.; et al. Phylogeny and Systematics of Demospongiae in Light of New Small-Subunit Ribosomal DNS (18S) Sequences. Integr. Comp. Biol. 2013, 53, 388–415. [Google Scholar] [CrossRef]
- Redmond, N.E.; van Soest, R.W.M.; Kelly, M.; Raleigh, J.; Travers, S.A.A. Reassessment of the classification of the Order Haplosclerida (Class Demospongiae, Phylum Porifera) using 18S rRNA gene sequence data. Mol. Phylogenet. Evol. 2007, 43, 344–352. [Google Scholar] [CrossRef]
- Fromont, J.; Sampey, A. Kimberley marine biota. Historical data: Sponges (Porifera). Rec. West Aust. Mus. Suppl. 2014, 84, 69–100. [Google Scholar] [CrossRef]
- Bryce, C.; Bryce, M.; Radford, B.T. Project methods and station geomorphology related to a multi-taxon survey (2009–2014) of the Kimberley. Rec. West Aust. Mus. Suppl. 2018, 85, 1–43. [Google Scholar] [CrossRef]
- Nozawa, Y.; Huang, Y.-S.; Hirose, E. Seasonality and lunar periodicity in the sexual reproduction of the coral-killing sponge, Terpios hoshinota. Coral Reefs 2016, 35, 1071–1081. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Wang, J.-T.; Chen, C.A. Larval release and rapid settlement of the coral-killing sponge, Terpios hoshinota, at Green Island, Taiwan. Mar. Biodivers. 2013, 43, 259–260. [Google Scholar] [CrossRef]
- Wang, J.-T.; Hirose, E.; Hsu, C.-M.; Chen, Y.-Y.; Meng, P.-J.; Chen, C.A. A Coral-Killing Sponge, Terpios hoshinota, Releases Larvae Harboring Cyanobacterial Symbionts: An Implication of Dispersal. Zool. Stud. 2012, 51, 314–320. [Google Scholar]
- Wang, J.-T.; Hsu, C.-M.; Kuo, C.-H.; Meng, P.-J.; Kao, S.-J.; Chen, C.A. Physiological Outperformance at the Morphologically-Transformed Edge of the Cyanobacteriosponge Terpios hoshinota (Suberitidae: Hadromerida) when Confronting Opponent Corals. PLoS ONE 2015, 10, e0131509. [Google Scholar] [CrossRef] [PubMed]
- Yomogida, M.; Mizuyama, M.; Kubomura, T.; Reimer, J.D. Disappearance and Return of an Outbreak of the Coral-killing Cyanobacteriosponge Terpios hoshinota in Southern Japan. Zool. Stud. 2017, 56, 1–10. [Google Scholar]
- Richards, Z.T.; Garcia, R.A.; Wallace, C.C.; Rosser, N.L.; Muir, P.R. A diverse assemblage of reef corals thriving in a dynamic intertidal reef setting (Bonaparte Archipelago, Kimberley, Australia). PLoS ONE 2015, 10, e0117791. [Google Scholar] [CrossRef] [PubMed]
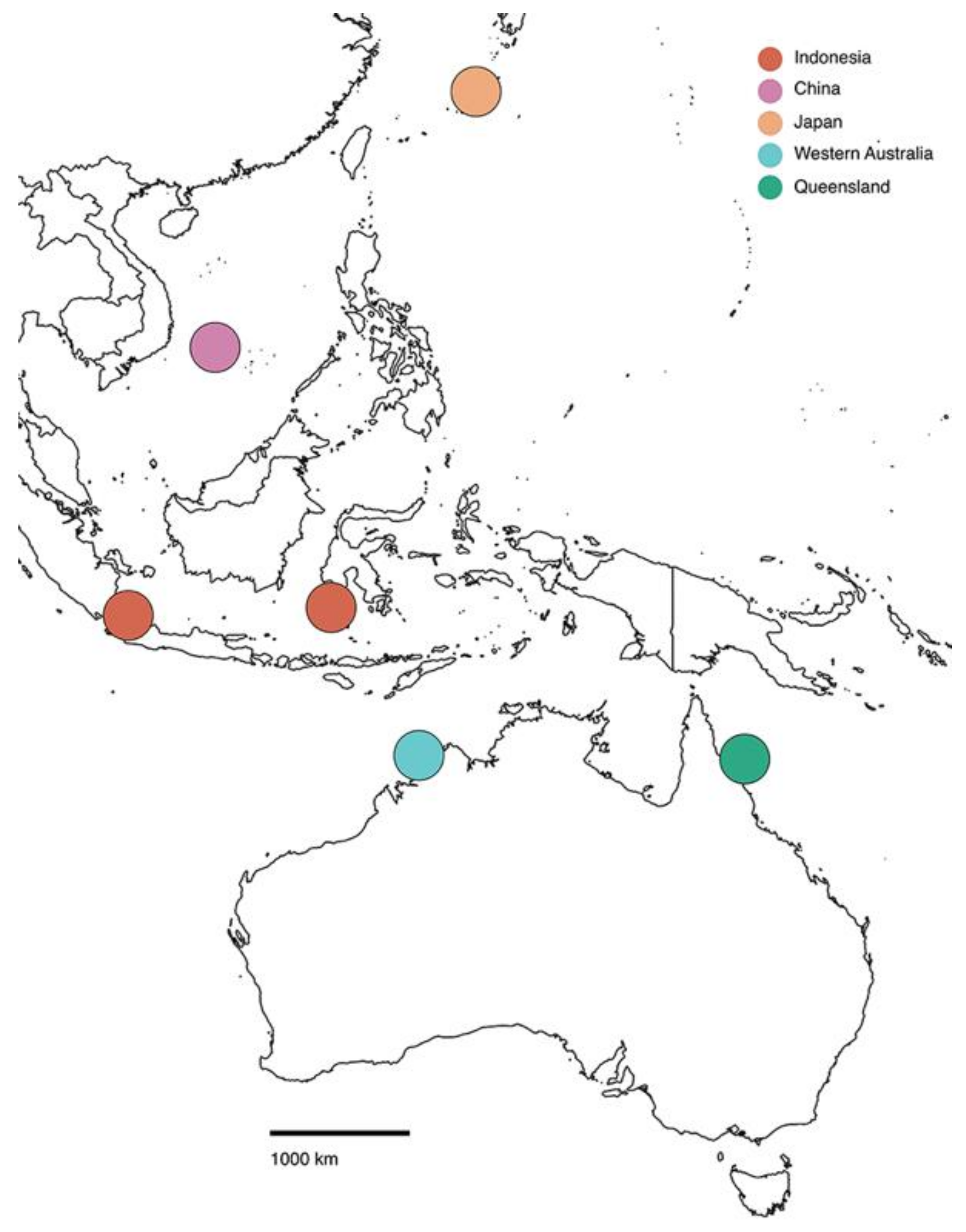
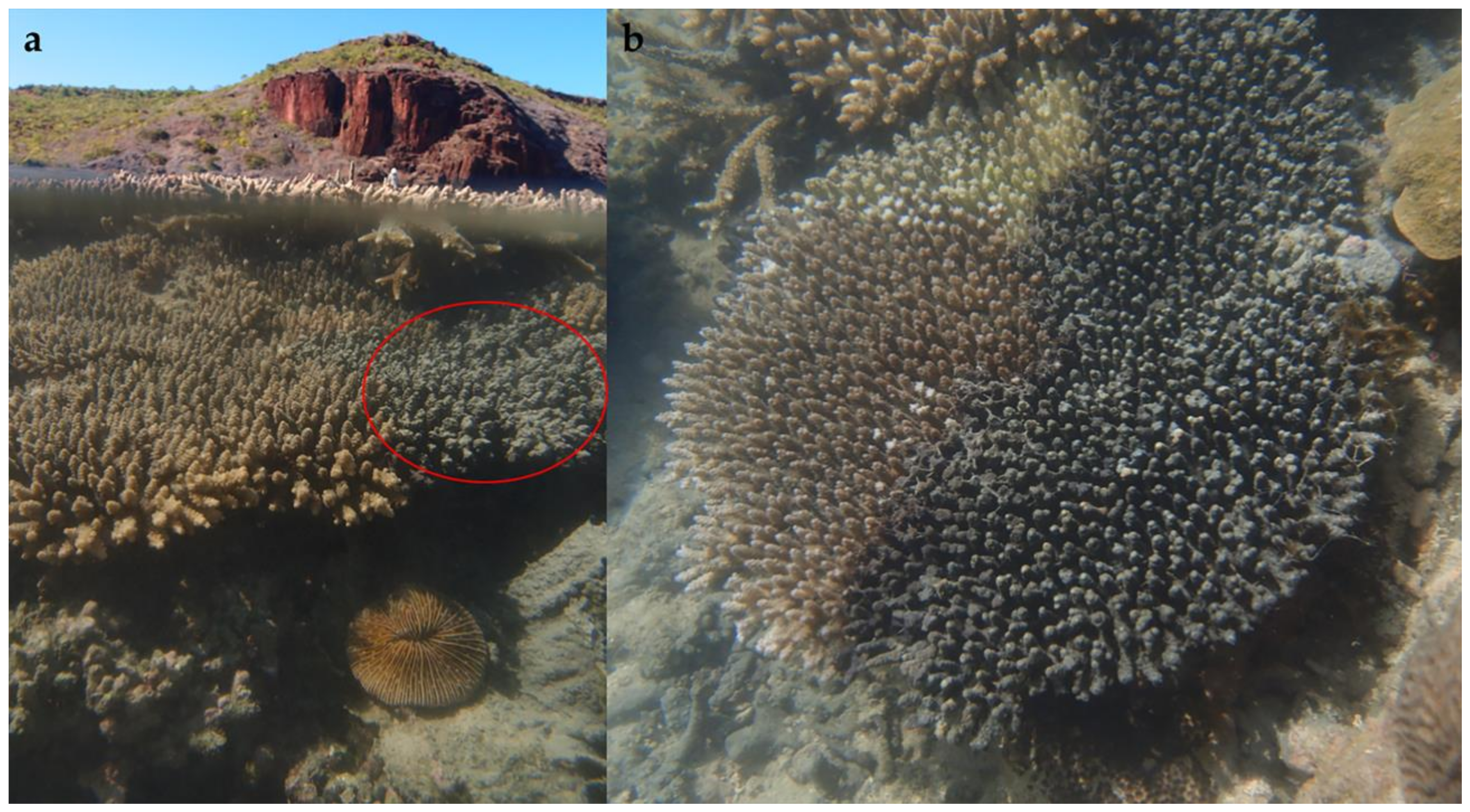
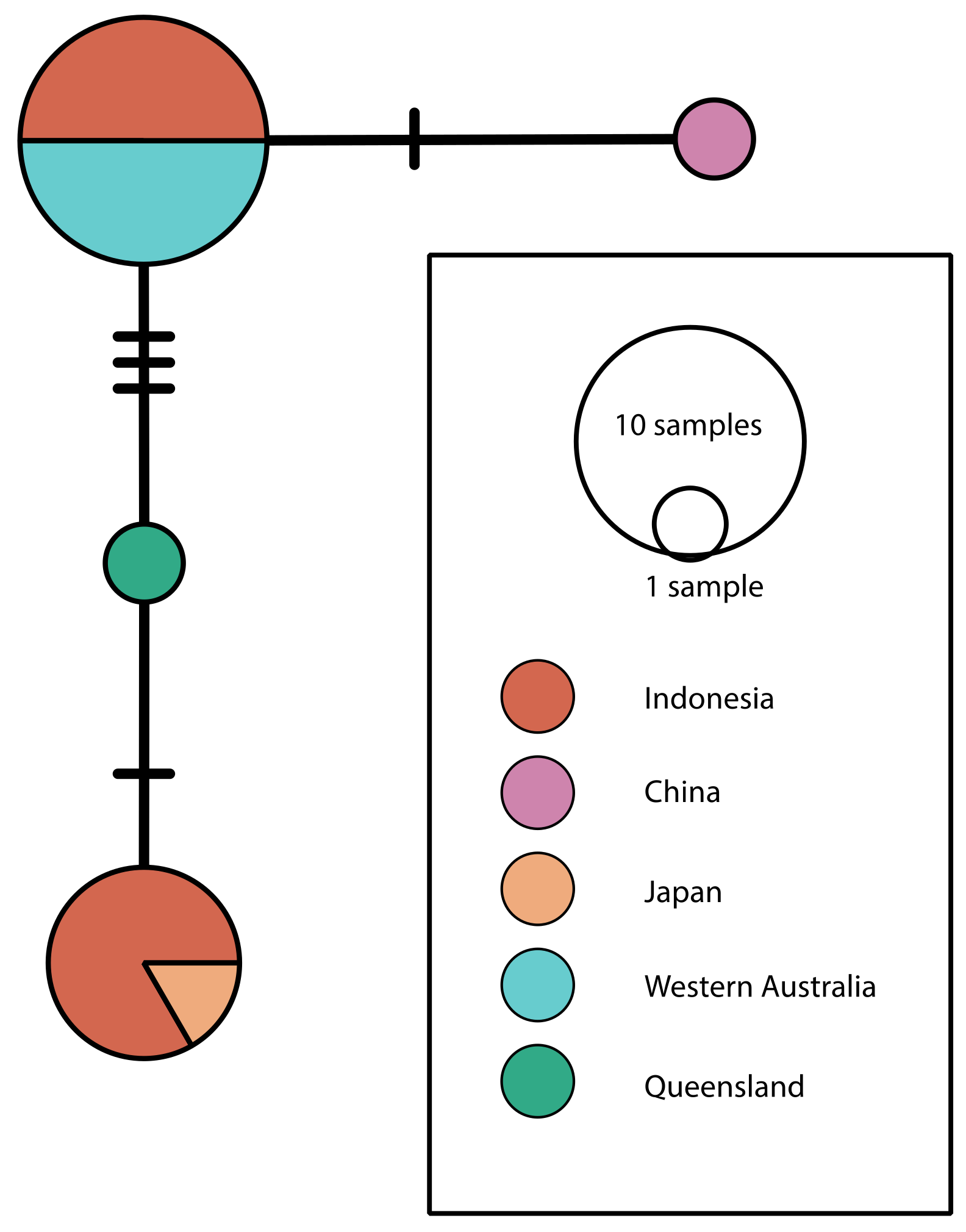
| Identifier | Source | Location | Depth (m) | COI Accession | 18S Accession |
|---|---|---|---|---|---|
| WAMZ83368 | This study | Berthier Island, Kimberley, WA | 0 | MN507874 | MN493758 |
| WAMZ83369 | This study | Berthier Island, Kimberley, WA | 0 | MN507875 | MN4937589 |
| WAMZ83370 | This study | Berthier Island, Kimberley, WA | 0 | MN507876 | - |
| WAMZ83371 | This study | Berthier Island, Kimberley, WA | 0 | MN507877 | - |
| WAMZ97242 | This study | West Montalivet Island, Kimberley, WA | 0 | MN507878 | - |
| QMG331910 | This study | Okinoerabu Is., Japan | 1 | MN507872 | - |
| QMG331911 | This study | Lizard Island, GBR, QLD, AUS | 1 | MN507873 | - |
| haplotype C1 | Lui, G., GenBank | South China Sea | Not provided | KJ008098 | - |
| haplotype C2 | van der Ent et al., 2016 [14] | Thousand Islands, Banten IND | Not provided | KP764915 | - |
| RMHN POR8730 | van der Ent et al., 2016 [14] | Palau Badi, South Sulawesi, IND | 6 | KP764915 | - |
| RMHN POR8731 | van der Ent et al., 2016 [14] | Palau Badi, South Sulawesi, IND | 6 | KP764915 | - |
| RMHN POR8732 | van der Ent et al., 2016 [14] | Palau Badi, South Sulawesi, IND | 6 | KP764915 | - |
| RMNH POR8736 | van der Ent et al., 2016 [14] | Kapoposang, South Sulawesi, IND | 3 | KP764915 | - |
| RMNH POR8729 | van der Ent et al., 2016 [14] | Samalona, South Sulawesi, IND | 3 | KP764916 | - |
| RMNH POR8733 | van der Ent et al., 2016 [14] | Lankai, South Sulawesi, IND | 3 | KP764916 | - |
| RMNH POR8734 | van der Ent et al., 2016 [14] | Lankai, South Sulawesi, IND | 3 | KP764916 | - |
| RMNH POR8735 | van der Ent et al., 2016 [14] | Lankai, South Sulawesi, IND | 3 | KP764916 | - |
| haplotype C3 | van der Ent et al., 2016 [14] | Lankai, South Sulawesi, IND | 3 | KP764916 | - |
| Primer Name | Primer Sequence | Program | Reference |
|---|---|---|---|
| COI SpongeCOI-F1/ jgHCO2198 | 5′-AGATAGGDACWGCNTTTA-3 5′-TAIACYTCIGGRTGICCRAARAAYCA-3′ | 94 °C 3 mi (94 °C 30 s, 41 °C 30 s, 72 °C 60 s) × 35, 72 °C 5 mi | Vargas et al. 2015 [34] Geller et al. 2013 [35] |
| SpongeCOI-F1/ dgHCO2198 | 5′-AGATAGGDACWGCNTTTA-3′ 5′-TAAACTTCAGGGTGACCAAARAAYCA-3′ | 94 °C 3 mi (94 °C 30 s, 49–35 °C (TD) 30 s, 72 °C 60 s) × 15, (94 °C 30 s, 41 °C 30 s, 60 °C 60 s) × 30, 72 °C 5 mi | Vargas et al. 2015 [34] Meyer et al. 2005 [36] |
| 18s SP18aF/ 600R18S | 5′-CCTGCCAGTAGTCATATGCTT-3′ 5′-CGAGCTTTTTAACTGCAA-3′ | 94 °C 5 mi (94 °C 60s, 49 °C 30 s, 72 °C 60–150 s) × 30, 72 °C 10 mi | Redmond et al. 2013 [37] Redmond et al. 2007 [38] |
| 400F18S/ 1350R18S | 5′-CCTGAGAAACGGCTACCACA-3′ 5′-CGGGACTAGTTAGCAGGTTAA-3′ | 94 °C 5 mi (94 °C 60s, 48 °C 30 s, 72 °C 60 s) × 30, 72 °C 10 mi | Redmond et al. 2007 [38] Redmond et al. 2007 [38] |
| 1200F18S/ SP18gR | 5′-TAATTTGACTCAACACGGG-3′ 5′-CCTTGTTACGACTTTTACTTCCTC-3′ | 94 °C 5 mi (94 °C 60 s, 48 °C 30 s, 72 °C 60 s) × 30, 72 °C 10 mi | Redmond et al. 2007 [38] Redmond et al. 2013 [37] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fromont, J.; Richards, Z.T.; Wilson, N.G. First Report of the Coral-Killing Sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A New Threat to Kimberley Coral Reefs? Diversity 2019, 11, 184. https://doi.org/10.3390/d11100184
Fromont J, Richards ZT, Wilson NG. First Report of the Coral-Killing Sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A New Threat to Kimberley Coral Reefs? Diversity. 2019; 11(10):184. https://doi.org/10.3390/d11100184
Chicago/Turabian StyleFromont, Jane, Zoe T. Richards, and Nerida G. Wilson. 2019. "First Report of the Coral-Killing Sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A New Threat to Kimberley Coral Reefs?" Diversity 11, no. 10: 184. https://doi.org/10.3390/d11100184
APA StyleFromont, J., Richards, Z. T., & Wilson, N. G. (2019). First Report of the Coral-Killing Sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A New Threat to Kimberley Coral Reefs? Diversity, 11(10), 184. https://doi.org/10.3390/d11100184






