Analysis of Lifetime Mortality Trajectories in Wildlife Disease Research: BaSTA and Beyond
Abstract
1. Introduction
2. Materials and Methods
2.1. Ecological Data
2.2. Diagnostic Tests
2.3. Analysis
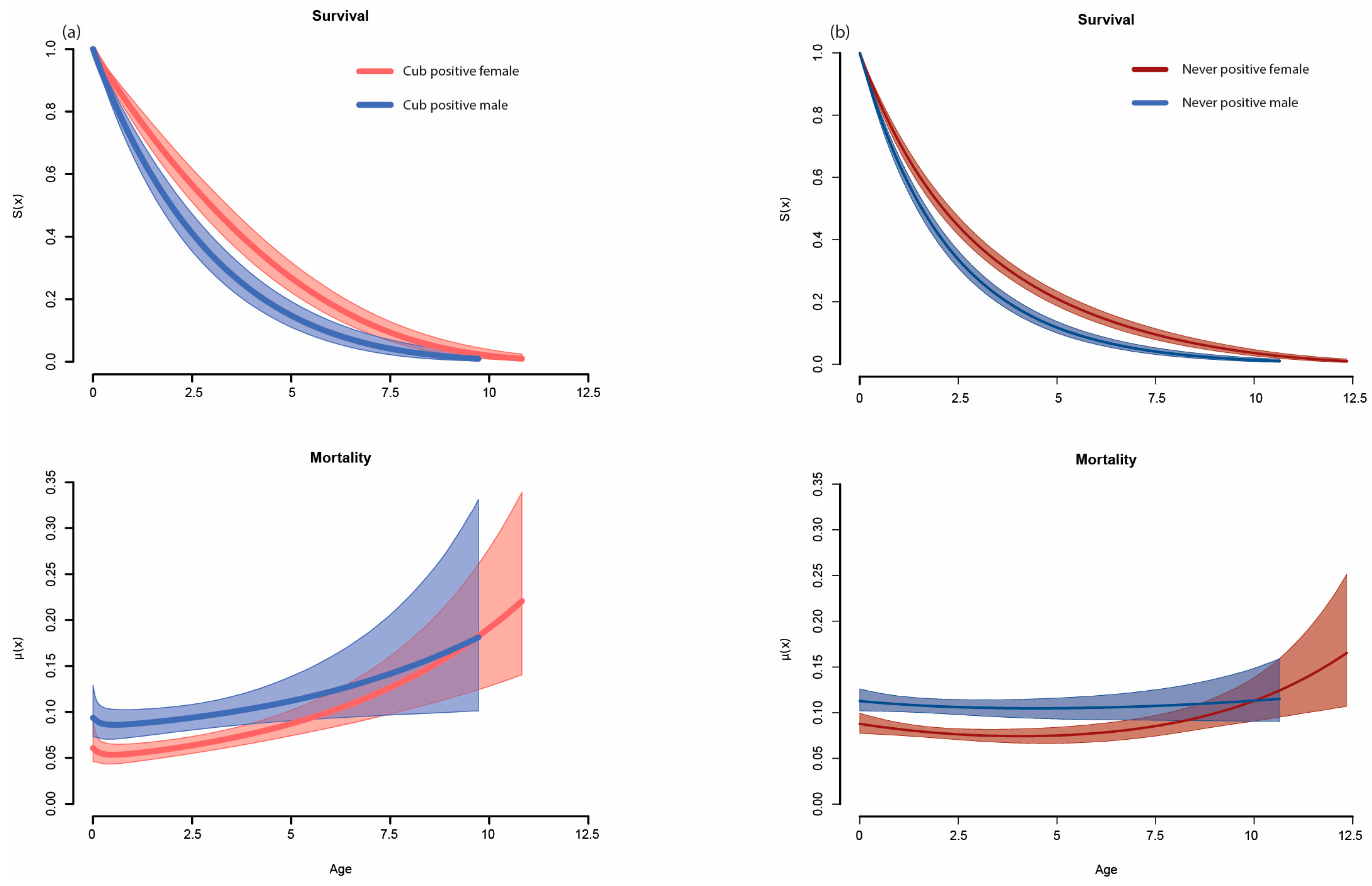
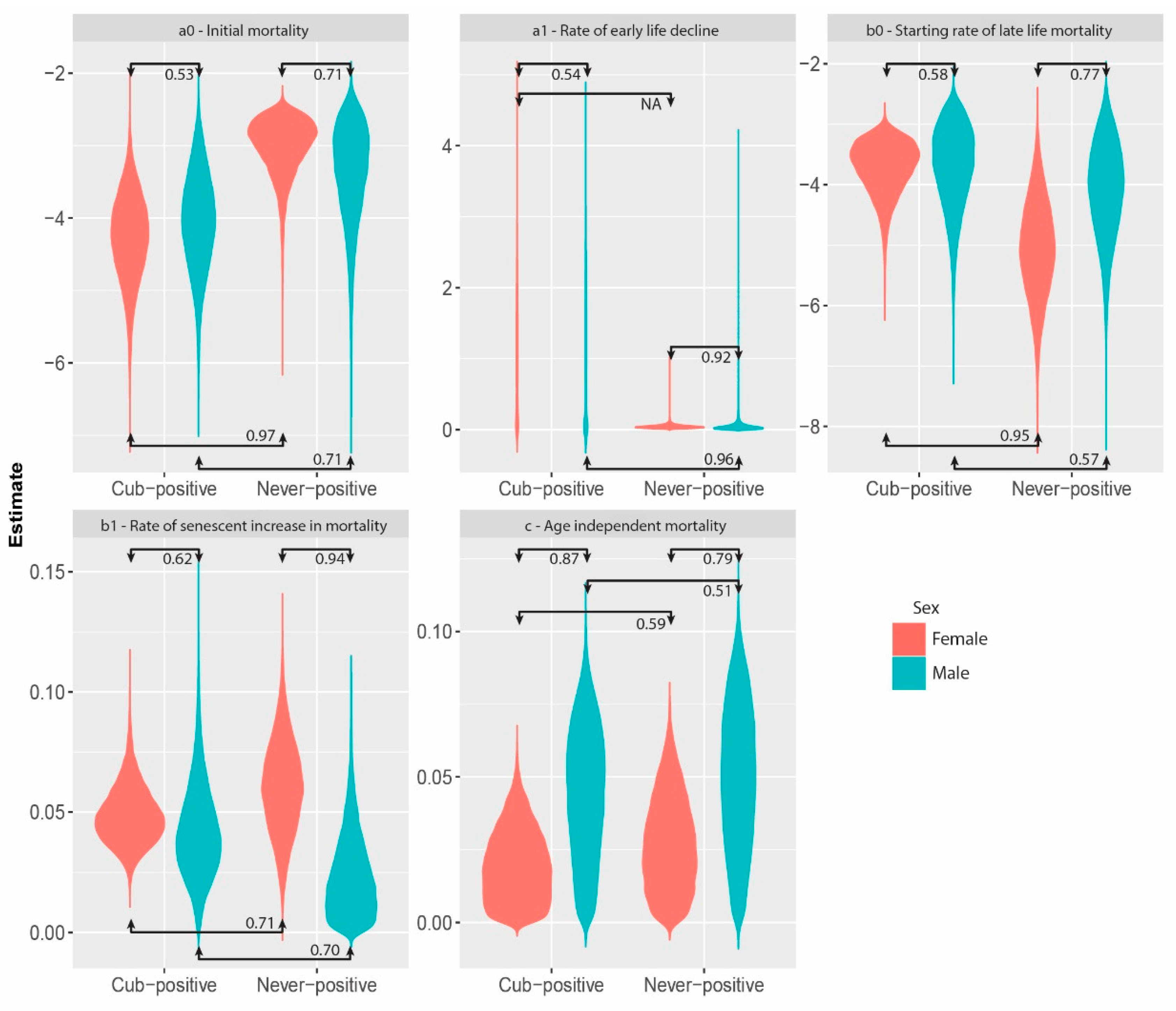
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Ethics Statement
Conflicts of Interest
References
- Delahay, R.J.; Smith, G.C.; Hutchings, M.R. The science of wildlife disease management. In Management of Disease in Wild Mammals; Springer: Tokyo, Japan, 2009; pp. 1–8. [Google Scholar]
- Gortázar, C.; Ferroglio, E.; Höfle, U.; Frölich, K.; Vicente, J. Diseases shared between wildlife and livestock: A European perspective. Eur. J. Wildl. Res. 2007, 53, 241–256. [Google Scholar] [CrossRef]
- Wiethoelter, A.K.; Beltrán-Alcrudo, D.; Kock, R.; Mor, S.M. Global trends in infectious diseases at the wildlife-livestock interface. Proc. Natl. Acad. Sci. USA 2015, 112, 9662–9667. [Google Scholar] [CrossRef] [PubMed]
- Heisey, D.M.; Joly, D.O.; Messier, F. The fitting of general force-of-infection models to wildlife disease prevalence data. Ecology 2006, 87, 2356–2365. [Google Scholar] [CrossRef]
- Samuel, M.D.; Woodworth, B.L.; Atkinson, C.T.; Hart, P.J.; Lapointe, D.A. Avian malaria in Hawaiian forest birds: Infection and population impacts across species and elevations. Ecosphere 2015, 6, 1–21. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Briggs, C.J.; Daszak, P. The ecology and impact of chytridiomycosis: An emerging disease of amphibians. Trends Ecol. Evol. 2010, 25, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Frick, W.F.; Pollock, J.F.; Hicks, A.C.; Langwig, K.E.; Reynolds, D.S.; Turner, G.G.; Butchkoski, C.M.; Kunz, T.H. An emerging disease causes regional population collapse of a common North American bat species. Science 2010, 329, 679–682. [Google Scholar] [CrossRef]
- van Riper, C.; van Riper, S.G.; Goff, M.L.; Laird, M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol. Monogr. 1986, 56, 327–344. [Google Scholar] [CrossRef]
- Berger, L.; Roberts, A.A.; Voyles, J.; Longcore, J.E.; Murray, K.A.; Skerratt, L.F. History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 2016, 19, 89–99. [Google Scholar] [CrossRef]
- Samuel, M.D.; Storm, D.J. Chronic wasting disease in white-tailed deer: Infection, mortality, and implications for heterogeneous transmission. Ecology 2016, 97, 3195–3205. [Google Scholar] [CrossRef]
- Wilkinson, D.; Smith, G.C.; Delahay, R.J.; Rogers, L.M.; Cheeseman, C.L.; Clifton-Hadley, R.S. The effects of bovine tuberculosis (Mycobacterium bovis) on mortality in a badger (Meles meles) population in England. J. Zool. 2000, 250, 389–395. [Google Scholar] [CrossRef]
- Fontana, L.; Kennedy, B.K.; Longo, V.D.; Seals, D.; Melov, S. Medical research: Treat ageing. Nature 2014, 511, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. The future of ageing. Nature 2000, 408, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Haas, G.P.; Delongchamps, N.; Brawley, O.W.; Wang, C.Y.; de la Roza, G. The worldwide epidemiology of prostate cancer: Perspectives from autopsy studies. Can. J. Urol. 2008, 15, 3866–3871. [Google Scholar] [PubMed]
- Jorgenson, J.T.; Festa-Bianchet, M.; Gaillard, J.-M.; Wishart, W.D. Effects of age, sex, disease, and density on survival of bighorn sheep. Ecology 1997, 78, 1019–1032. [Google Scholar] [CrossRef]
- Larsen, D.G.; Gauthier, D.A.; Markel, R.L. Causes and rate of moose mortality in the Southwest Yukon. J. Wildl. Manag. 1989, 53, 548–557. [Google Scholar] [CrossRef]
- Koons, D.N.; Gamelon, M.; Gaillard, J.-M.; Aubry, L.M.; Rockwell, R.F.; Klein, F.; Choquet, R.; Gimenez, O. Methods for studying cause-specific senescence in the wild. Methods Ecol. Evol. 2014, 5, 924–933. [Google Scholar] [CrossRef]
- Reid, J.M.; Bignal, E.M.; Bignal, S.; McCracken, D.I.; Monaghan, P. Age-specific reproductive performance in red-billed choughs Pyrrhocorax pyrrhocorax: Patterns and processes in a natural population. J. Anim. Ecol. 2003, 72, 765–776. [Google Scholar] [CrossRef]
- Reimers, E.; Holmengen, N.; Mysterud, A. Life-history variation of wild reindeer (Rangifer tarandus) in the highly productive North Ottadalen region, Norway. J. Zool. 2005, 265, 53–62. [Google Scholar] [CrossRef]
- Chen, G.H.; Wang, Y.J.; Wang, X.M.; Zhou, J.N.; Liu, R.Y. Effect of aging on species-typical behaviors in senescence-accelerated mouse. Physiol. Behav. 2005, 85, 536–545. [Google Scholar] [CrossRef]
- Angelier, F.; Weimerskirch, H.; Dano, S.; Chastel, O. Age, experience and reproductive performance in a long-lived bird: A hormonal perspective. Behav. Ecol. Sociobiol. 2007, 61, 611–621. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Evolutionary theories of aging: Confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Nat. 1998, 152, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Nussey, D.H.; Froy, H.; Lemaitre, J.-F.; Gaillard, J.-M.; Austad, S.N. Senescence in natural populations of animals: Widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 2013, 12, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Smith, G.C.; Delahay, R.J.; Bailey, T.; McDonald, R.A.; Hodgson, D. Multi-state modelling reveals sex-dependent transmission, progression and severity of tuberculosis in wild badgers. Epidemiol. Infect. 2013, 141, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Nussey, D.H.; Coulson, T.; Festa-Bianchet, M.; Gaillard, J.-M. Measuring senescence in wild animal populations: Towards a longitudinal approach. Funct. Ecol. 2008, 22, 393–406. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Scheuerlein, A. Biological implications of the Weibull and Gompertz models of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B69–B76. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.L. The analysis of survival (mortality) data: Fitting Gompertz, Weibull, and logistic functions. Mech. Ageing Dev. 1994, 74, 15–33. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new. Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar]
- Pinder, J.E.; Wiener, J.G.; Smith, M.H. The Weibull distribution: A new method of summarizing survivorship data. Ecology 1978, 59, 175–179. [Google Scholar] [CrossRef]
- Colchero, F.; Clark, J.S. Bayesian inference on age-specific survival for censored and truncated data. J. Anim. Ecol. 2012, 81, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Makeham, W.M. On the law of mortality. J. Inst. Actuar. 1867, 13, 325–358. [Google Scholar] [CrossRef]
- Cam, E.; Aubry, L.M.; Authier, M. The conundrum of heterogeneities in life history studies. Trends Ecol. Evol. 2016, 31, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, J.W.; Manton, K.G.; Stallard, E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography 1979, 16, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, S.D. Pletcher model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 1999, 12, 430–439. [Google Scholar] [CrossRef]
- Jones, O.R.; Scheuerlein, A.; Salguero-Gómez, R.; Camarda, C.G.; Schaible, R.; Casper, B.B.; Dahlgren, J.P.; Ehrlén, J.; García, M.B.; Menges, E.S.; et al. Diversity of ageing across the tree of life. Nature 2014, 505, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, J.W.; Carey, J.R.; Christensen, K.; Johnson, T.E.; Yashin, A.I.; Holm, N.V.; Iachine, I.A.; Kannisto, V.; Khazaeli, A.A.; Liedo, P.; et al. Biodemographic trajectories of longevity. Science 1998, 280, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E.; Scheuerlein, A. Comparison of aging-related mortality among birds and mammals. Exp. Gerontol. 2001, 36, 845–857. [Google Scholar] [CrossRef]
- Barthold, J.A.; Loveridge, A.J.; Macdonald, D.W.; Packer, C.; Colchero, F. Bayesian estimates of male and female African lion mortality for future use in population management. J. Appl. Ecol. 2016, 53, 295–304. [Google Scholar] [CrossRef]
- Caughley, G. Mortality patterns in mammals. Ecology 1966, 47, 906–918. [Google Scholar] [CrossRef]
- Siler, W. A competing-risk model for animal mortality. Ecology 1979, 60, 750–757. [Google Scholar] [CrossRef]
- Klutke, G.A.; Kiessler, P.C.; Wortman, M.A. A critical look at the bathtub curve. IEEE Trans. Reliab. 2003, 52, 125–129. [Google Scholar] [CrossRef]
- Bebbington, M.; Lai, C.-D.; Zitikis, R. Modeling human mortality using mixtures of bathtub shaped failure distributions. J. Theor. Biol. 2007, 245, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Snoke, M.S.; Promislow, D.E.L. Quantitative genetic tests of recent senescence theory: Age-specific mortality and male fertility in Drosophila melanogaster. Heredity 2003, 91, 546–556. [Google Scholar] [CrossRef]
- McDonald, J.L.; Smith, G.C.; McDonald, R.A.; Delahay, R.J.; Hodgson, D. Mortality trajectory analysis reveals the drivers of sex-specific epidemiology in natural wildlife-disease interactions. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140526. [Google Scholar] [CrossRef]
- Delahay, R.J.; Walker, N.; Smith, G.S.; Wilkinson, D.; Clifton-Hadley, R.S.; Cheeseman, C.L.; Tomlinson, A.J.; Chambers, M.A. Long-term temporal trends and estimated transmission rates for Mycobacterium bovis infection in an undisturbed high-density badger (Meles meles) population. Epidemiol. Infect. 2013, 141, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.A.; Woodroffe, R.; Cox, D.R.; Bourne, F.J.; Cheeseman, C.L.; Clifton-Hadley, R.S.; Wei, G.; Gettinby, G.; Gilks, P.; Jenkins, H.; et al. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 2006, 439, 843–846. [Google Scholar] [CrossRef]
- Gallagher, J.; Clifton-Hadley, R.S. Tuberculosis in badgers; a review of the disease and its significance for other animals. Res. Vet. Sci. 2000, 69, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Colchero, F.; Jones, O.R.; Rebke, M. BaSTA: An R package for Bayesian estimation of age-specific survival from incomplete mark-recapture/recovery data with covariates. Methods Ecol. Evol. 2012, 3, 466–470. [Google Scholar] [CrossRef]
- Colchero, F.; Aliaga, A.E.; Jones, O.R.; Conde, D.A. Individual heterogeneity determines sex differences in mortality in a monogamous bird with reversed sexual dimorphism. J. Anim. Ecol. 2017, 86, 899–970. [Google Scholar] [CrossRef] [PubMed]
- Tidière, M.; Gaillard, J.-M.; Müller, D.W.H.; Lackey, L.B.; Gimenez, O.; Clauss, M.; Lemaître, J.-F. Does sexual selection shape sex differences in longevity and senescence patterns across vertebrates? A review and new insights from captive ruminants. Evolution 2015, 69, 3123–3140. [Google Scholar] [CrossRef]
- Lemaître, J.-F.; Gaillard, J.-M.; Lackey, L.B.; Clauss, M.; Müller, D.W.H. Comparing free-ranging and captive populations reveals intra-specific variation in aging rates in large herbivores. Exp. Gerontol. 2013, 48, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci. Biobehav. Rev. 2000, 24, 627–638. [Google Scholar] [CrossRef]
- Møller, A.P. Advantages and disadvantages of coloniality in the swallow, Hirundo rustica. Anim. Behav. 1987, 35, 819–832. [Google Scholar] [CrossRef]
- Zuk, M.; McKean, K.A. Sex differences in parasite infections: Patterns and processes. Int. J. Parasitol. 1996, 26, 1009–1024. [Google Scholar] [CrossRef]
- Berger, V.; Lemaître, J.-F.; Dupont, P.; Allainé, D.; Gaillard, J.-M.; Cohas, A. Age-specific survival in the socially monogamous alpine marmot (Marmota marmota): Evidence of senescence. J. Mammal. 2016, 97, 992–1000. [Google Scholar] [CrossRef]
- Baudisch, A. The pace and shape of ageing. Methods Ecol. Evol. 2011, 2, 375–382. [Google Scholar] [CrossRef]
- McDonald, J.L.; Hodgson, D.J. Prior precision, prior accuracy, and the estimation of disease prevalence using imperfect diagnostic tests. Front. Vet. Sci. 2018, 5, 83. [Google Scholar] [CrossRef]
- McDonald, J.L.; Robertson, A.; Silk, M.J. Wildlife disease ecology from the individual to the population: Insights from a long-term study of a naturally infected European badger population. J. Anim. Ecol. 2018, 87, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Delahay, R.J.; Langton, S.; Smith, G.C.; Clifton-Hadley, R.S.; Cheeseman, C.L. The spatio-temporal distribution of Mycobacterium bovis (bovine tuberculosis) infection in a high-density badger population. J. Anim. Ecol. 2000, 69, 428–441. [Google Scholar] [CrossRef]
- Gallagher, J.; Horwill, D.M. A selective oleic acid albumin agar medium for the cultivation of Mycobacterium bovis. Epidemiol. Infect. 1977, 79, 155–160. [Google Scholar] [CrossRef]
- Goodger, J.; Nolan, A.; Russell, W.P.; Dalley, D.J.; Thorns, C.J.; Stuart, F.A.; Croston, P.; Newell, D.G. Serodiagnosis of Mycobacterium bovis infection in badgers: Development of an indirect ELISA using a 25 kDa antigen. Vet. Rec. 1994, 135, 82–85. [Google Scholar] [CrossRef]
- Chambers, M.A.; Crawshaw, T.; Waterhouse, S.; Delahay, R.; Hewinson, R.G.; Lyashchenko, K.P. Validation of the BrockTB stat-pak assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 2008, 46, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Hadley, R.S.; Sayers, A.R.; Stock, M.P. Evaluation of an ELISA for Mycobacterium bovis infection in badgers (Meles meles). Vet. Rec. 1995, 137, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Drewe, J.A.; Tomlinson, A.J.; Walker, N.J.; Delahay, R.J. Diagnostic accuracy and optimal use of three tests for tuberculosis in live badgers. PLoS ONE 2010, 5, e11196. [Google Scholar] [CrossRef] [PubMed]
- Buzdugan, S.N.; Chambers, M.A.; Delahay, R.J.; Drewe, J.A. Diagnosis of tuberculosis in groups of badgers: An exploration of the impact of trapping efficiency, infection prevalence and the use of multiple tests. Epidemiol. Infect. 2016, 144, 1717–1727. [Google Scholar] [CrossRef]
- Wawegama, N.K.; Markham, P.F.; Kanci, A.; Schibrowski, M.; Oswin, S.; Barnes, T.S.; Firestone, S.M.; Mahony, T.J.; Browning, G.F. Evaluation of an IgG enzyme-linked immunosorbent assay as a serological assay for detection of mycoplasma bovis infection in feedlot cattle. J. Clin. Microbiol. 2016, 54, 1269–1275. [Google Scholar] [CrossRef]
- Rogers, L.M.; Cheeseman, C.L.; Mallinson, P.J.; Clifton-Hadley, R. The demography of a high-density badger (Meles meles) population in the west of England. J. Zool. 1997, 242, 705–728. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing. R Found. R Found. Stat. Comput. 2011. Available online: https://www.r-project.org/ (accessed on 12 February 2019).
- Kalbfleisch, J.D.; Prentice, R.L. The Statistical Analysis of Failure Time Data; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; ISBN 9781118032985. [Google Scholar]
- Gelman, A.; Carlin, J.B.B.; Stern, H.S.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B.B. Bayesian Data Analysis, 3rd ed.; Texts in Statistical Science; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781439840955. [Google Scholar]
- Kass, R.E.; Raftery, A.E. Bayes factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B 2002, 64, 583–639. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van der Linde, A. The deviance information criterion: 12 years on. J. R. Stat. Soc. Ser. B 2014, 76, 485–493. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On information and sufficiency. Ann. Math. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- McCulloch, R.E. Local model influence. J. Am. Stat. Assoc. 1989, 84, 473–478. [Google Scholar] [CrossRef]
- Larson, S.M.; Colchero, F.; Jones, O.R.; Williams, L.; Fernandez-Duque, E. Age and sex-specific mortality of wild and captive populations of a monogamous pair-bonded primate (Aotus azarae). Am. J. Primatol. 2016, 78, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Bronikowski, A.M.; Altmann, J.; Brockman, D.K.; Cords, M.; Fedigan, L.M.; Pusey, A.; Stoinski, T.; Morris, W.F.; Strier, K.B.; Alberts, S.C. Aging in the natural world: Comparative data reveal similar mortality patterns across primates. Science 2011, 311, 1325–1328. [Google Scholar] [CrossRef] [PubMed]
- Beirne, C.; Delahay, R.; Young, A. Sex differences in senescence: The role of intra-sexual competition in early adulthood. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151086. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.P.; Miller, D.C.; Schafer, W.D. Gender differences in risk taking: A meta-analysis. Psychol. Bull. 1999, 125, 367. [Google Scholar] [CrossRef]
- Delahay, R.J.; Walker, N.J.; Forrester, G.J.; Harmsen, B.; Riordan, P.; Macdonald, D.W.; Newman, C.; Cheeseman, C.L. Demographic correlates of bite wounding in Eurasian badgers, Meles meles L., in stable and perturbed populations. Anim. Behav. 2006, 71, 1047–1055. [Google Scholar] [CrossRef]
- Greiner, S.; Nagy, M.; Mayer, F.; Knörnschild, M.; Hofer, H.; Voigt, C.C. Sex-biased senescence in a polygynous bat species. Ethology 2014, 120, 197–205. [Google Scholar] [CrossRef]
- Descamps, S.; Boutin, S.; Berteaux, D.; Gaillard, J.M. Age-specific variation in survival, reproductive success and offspring quality in red squirrels: Evidence of senescence. Oikos 2008, 117, 1406–1416. [Google Scholar] [CrossRef]
- Lemaitre, J.F.; Gaillard, J.M. Male survival patterns do not depend on male allocation to sexual competition in large herbivores. Behav. Ecol. 2012, 24, 421–428. [Google Scholar] [CrossRef]
- Noonburg, E.G.; Chen, A.; Shima, J.S.; Swearer, S.E. Demographic heterogeneity and the dynamics of open populations. Ecology 2015, 96, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, J.P.; Colchero, F.; Jones, O.R.; Øien, D.-I.; Moen, A.; Sletvold, N. Actuarial senescence in a long-lived orchid challenges our current understanding of ageing. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161217. [Google Scholar] [CrossRef] [PubMed]
- Kynaston, S.; Neal, E.; Cheeseman, C. Badgers. J. Anim. Ecol. 2006, 65, 533. [Google Scholar] [CrossRef]
- Dalley, D.; Davé, D.; Lesellier, S.; Palmer, S.; Crawshaw, T.; Hewinson, R.G.; Chambers, M. Development and evaluation of a gamma-interferon assay for tuberculosis in badgers (Meles meles). Tuberculosis 2008, 88, 235–243. [Google Scholar] [CrossRef]
- Conn, P.B.; Cooch, E.G. Multistate capture-recapture analysis under imperfect state observation: An application to disease models. J. Appl. Ecol. 2009, 46, 486–492. [Google Scholar] [CrossRef]
- Pradel, R.; Hines, J.E.; Lebreton, J.-D.; Nichols, J.D. Capture-recapture survival models taking account of transients. Biometrics 1997, 53, 60–72. [Google Scholar] [CrossRef]
- Choquet, R.; Rouan, L.; Pradel, R. Program E-surge: A software application for fitting multievent models. In Modeling Demographic Processes in Marked Populations; Springer: Boston, MA, USA, 2009; pp. 845–865. [Google Scholar]
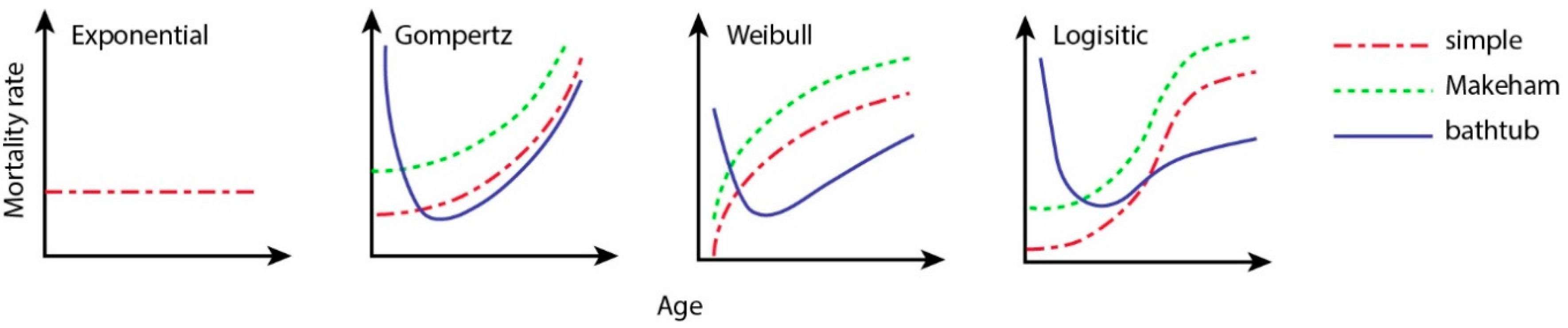
| Model | Parameters | ||
|---|---|---|---|
| Exponential | |||
| Gompertz [28] | |||
| Weibull [29] | |||
| Logistic [33] |
| Summary Statistic | Cub-Positive | Never-Positive |
|---|---|---|
| Total number badgers | 428 (M 191; F 237) | 1768 (M 833; F 935) |
| Number of known birth years | 428 | 1768 |
| Number of known death years | 13 | 323 |
| Total number of detections | 2515 | 7588 |
| Cub-Positive (in Rank Order by DIC) | Never-Positive | ||||||
|---|---|---|---|---|---|---|---|
| Model | Shape | DIC | DIC | Model | Shape | DIC | DIC |
| Gompertz | Bathtub | 4622 | 0 | Gompertz | Bathtub | 25,678 | 0 |
| Gompertz | Simple | 4642 | 20 | Exponential | Simple | 25,693 | 15 |
| Logistic | Bathtub | 4661 | 39 | Weibull | Bathtub | 25,695 | 17 |
| Weibull | Bathtub | 4669 | 47 | Weibull | Makeham | 25,954 | 276 |
| Weibull | Makeham | 4675 | 53 | Logistic | Makeham | 25,975 | 297 |
| Logistic | Makeham | 4682 | 60 | Logistic | Simple | 25,982 | 304 |
| Weibull | Simple | 4689 | 67 | Gompertz | Makeham | 26,004 | 326 |
| Logistic | Simple | 4697 | 75 | Logistic | Bathtub | 26,048 | 370 |
| Gompertz | Makeham | 4710 | 88 | Gompertz | Simple | 26,136 | 458 |
| Exponential | Simple | 4741 | 119 | Weibull | Simple | 26,235 | 557 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudson, D.W.; Delahay, R.; McDonald, R.A.; McKinley, T.J.; Hodgson, D.J. Analysis of Lifetime Mortality Trajectories in Wildlife Disease Research: BaSTA and Beyond. Diversity 2019, 11, 182. https://doi.org/10.3390/d11100182
Hudson DW, Delahay R, McDonald RA, McKinley TJ, Hodgson DJ. Analysis of Lifetime Mortality Trajectories in Wildlife Disease Research: BaSTA and Beyond. Diversity. 2019; 11(10):182. https://doi.org/10.3390/d11100182
Chicago/Turabian StyleHudson, Dave W., Richard Delahay, Robbie A. McDonald, Trevelyan J. McKinley, and Dave J. Hodgson. 2019. "Analysis of Lifetime Mortality Trajectories in Wildlife Disease Research: BaSTA and Beyond" Diversity 11, no. 10: 182. https://doi.org/10.3390/d11100182
APA StyleHudson, D. W., Delahay, R., McDonald, R. A., McKinley, T. J., & Hodgson, D. J. (2019). Analysis of Lifetime Mortality Trajectories in Wildlife Disease Research: BaSTA and Beyond. Diversity, 11(10), 182. https://doi.org/10.3390/d11100182