Genetic Characterization of Cleveland Bay Horse Breed
Abstract
1. Introduction
2. Materials and Methods
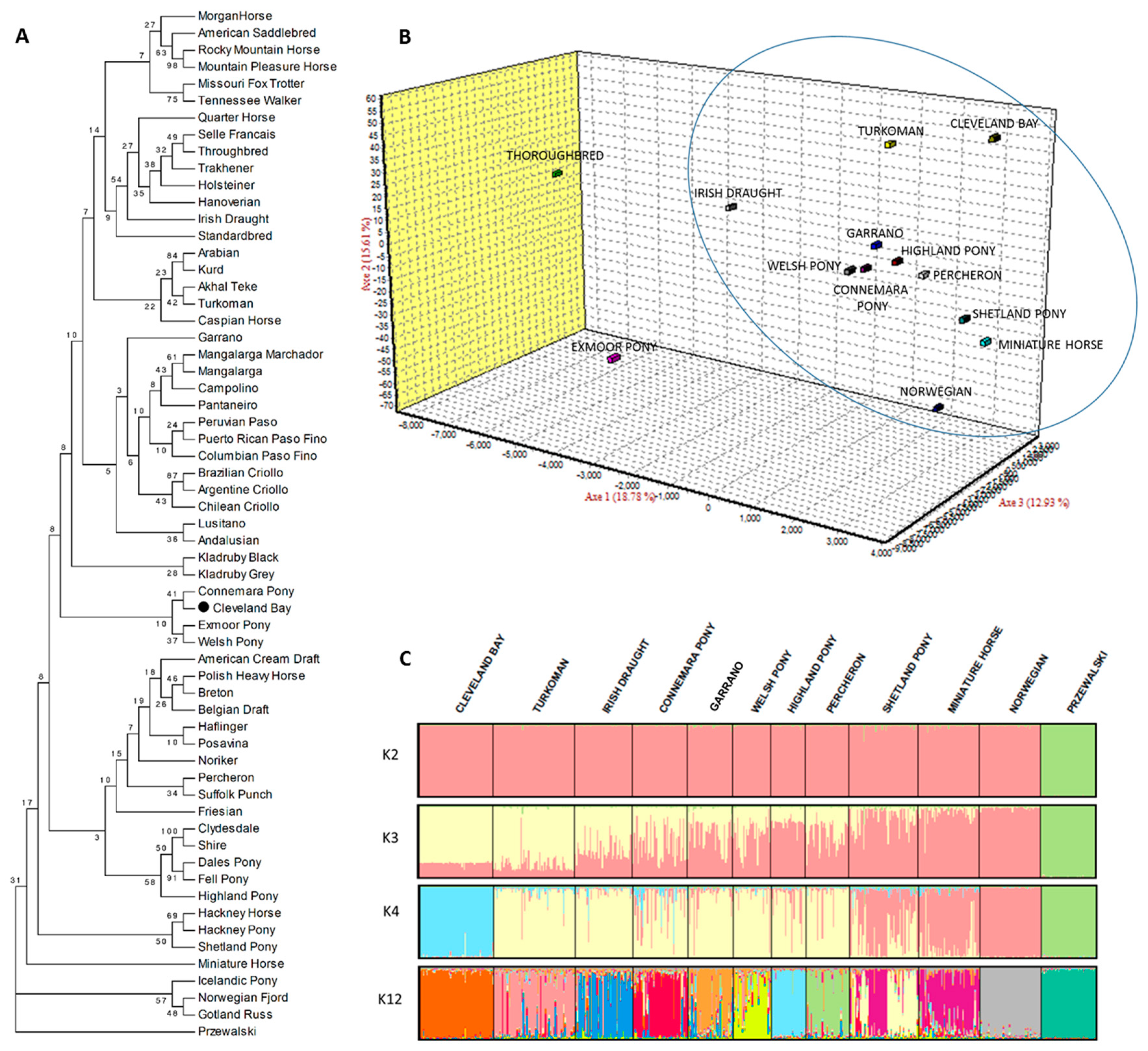
3. Results and discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Austen, C.; Corrie, S.; Swinney, N.J. The Complete Illustrated Encyclopedia of Horses; Metro Books: New York, NY, USA, 2008. [Google Scholar]
- Hendricks, B.L. International Encyclopedia of Horse Breeds; University of Oklahoma Press: Norman, OK, USA, 1995. [Google Scholar]
- Dixon, W.S. The Cleveland Bay Stud Book; Cleveland Bay Horse Society: Middlesbrough, UK, 1884. [Google Scholar]
- Sidney, S.; Sinclair, J.; Charles, W.; Blew, A. The Book of the Horse; Cassell & Company: Detroit, MI, USA, 1893. [Google Scholar]
- Edwards, E. The Encyclopedia of the Horse; Kindersley: New York, NY, USA, 1994. [Google Scholar]
- Cothran, E.; Santos, S.A.; Mazza, M.C.M.; Lear, T.L.; Sereno, J. Genetics of the Pantaneiro Horse of the Pantanal Region of Brazil. Genet. Mol. Biol. 1998, 21. [Google Scholar] [CrossRef]
- Cothran, E.G.; van Dyk, E. Genetic Analysis of Three South African Horse Breeds. J. S. Afr. Vet. Assoc. 1998, 69, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Juras, R.; Cothran, E.G.; Klimas, R. Genetic Analysis of Three Lithuanian Native Horse Breeds. Acta Agric. Scand. Sect. A Anim. Sci. 2003, 53, 180–185. [Google Scholar] [CrossRef]
- Achmann, R.; Curik, I.; Dovc, P.; Kavar, T.; Bodo, I.; Habe, F.; Marti, E.; Solkner, J.; Brem, G. Microsatellite Diversity, Population Subdivision and Gene Flow in the Lipizzan Horse. Anim. Genet. 2004, 35, 285–292. [Google Scholar] [CrossRef]
- Luis, C.; Bastos-Silveira, C.; Cothran, E.G.; Mdo, M.O. Iberian Origins of New World Horse Breeds. J. Hered. 2006, 97, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Luis, C.; Juras, R.; Oom, M.M.; Cothran, E.G. Genetic Diversity and Relationships of Portuguese and Other Horse Breeds Based on Protein and Microsatellite Loci Variation. Anim. Genet. 2007, 38, 20–27. [Google Scholar] [CrossRef]
- Plante, Y.; Vega-Pla, J.L.; Lucas, Z.; Colling, D.; de March, B.; Buchanan, F. Genetic Diversity in a Feral Horse Population from Sable Island, Canada. J. Hered. 2007, 98, 594–602. [Google Scholar] [CrossRef]
- Thirstrup, J.P.; Bach, L.A.; Loeschcke, V.; Pertoldi, C. Population Viability Analysis on Domestic Horse Breeds (Equus Caballus). J. Anim. Sci. 2009, 87, 3525–3535. [Google Scholar] [CrossRef]
- Notter, D.R. The Importance of Genetic Diversity in Livestock Populations of the Future. J. Anim. Sci. 1999, 77, 61–69. [Google Scholar] [CrossRef]
- Khanshour, A.; Conant, E.; Juras, R.; Cothran, E.G. Microsatellite Analysis of Genetic Diversity and Population Structure of Arabian Horse Populations. J. Hered. 2013, 104, 386–398. [Google Scholar] [CrossRef]
- Paetkau, D.; Waits, L.P.; Clarkson, P.L.; Craighead, L.; Strobeck, C. An Empirical Evaluation of Genetic Distance Statistics Using Microsatellite Data from Bear (Ursidae) Populations. Genetics 1997, 147, 1943. [Google Scholar] [PubMed]
- Felsenstein, J. Phylip (Phylogeny Inference Package); Version 3.6. Distributed by the Author; University of Washington: Seattle, WA, USA, 2004; Available online: http://evolution.genetics.washington.edu/phylip.html (accessed on 13 August 2017).
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mega4: Molecular Evolutionary Genetics Analysis (Mega) Software Version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.E.; Bannasch, D.L.; Petersen, J.L.; Gurr, J.; Bailey, E.; Binns, M.M.; Distl, O.; Guerin, G.; Hasegawa, T.; Hill, E.W.; et al. A High Density Snp Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies. PLoS Genet. 2012, 8, e1002451. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, F.; Bonhomme, F. Genetix 4.05, Windows Tm Software for Population Genetics. In Laboratorie Genome, Populations, Interactions; Universite Montpellier II: Montpellier, France, 1996–2004. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Jakobsson, M.; Rosenberg, N.A. Clumpp: A Cluster Matching and Permutation Program for Dealing with Label Switching and Multimodality in Analysis of Population Structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. Distruct: A Program for the Graphical Display of Population Structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software Structure: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; von Holdt, B.M. Structure Harvester: A Website and Program for Visualizing Structure Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Petersen, J.L.; Mickelson, J.R.; Cothran, E.G.; Andersson, L.S.; Axelsson, J.; Bailey, E.; Bannasch, D.; Binns, M.M.; Borges, A.S.; Brama, P.; et al. Genetic Diversity in the Modern Horse Illustrated from Genome-Wide Snp Data. PLoS ONE 2013, 8, e54997. [Google Scholar] [CrossRef]
- Ducro, B.J.; Windig, J.J.; Hellinga, I.; Bovenhuis, H. Genetic Diversity and Measures to Reduce Inbreeding in Friesian Horses. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, ASAS, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- Metzger, J.; Tonda, R.; Beltran, S.; Agueda, L.; Gut, M.; Distl, O. Next Generation Sequencing Gives an Insight into the Characteristics of Highly Selected Breeds Versus Non-Breed Horses in the Course of Domestication. BMC Genom. 2014, 15, 562. [Google Scholar] [CrossRef]
- Schubert, M.; Jonsson, H.; Chang, D.; der Sarkissian, C.; Ermini, L.; Ginolhac, A.; Albrechtsen, A.; Dupanloup, I.; Foucal, A.; Petersen, B.; et al. Prehistoric Genomes Reveal the Genetic Foundation and Cost of Horse Domestication. Proc. Natl. Acad. Sci. USA 2014, 111, E5661–E5669. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanshour, A.M.; Hempsey, E.K.; Juras, R.; Cothran, E.G. Genetic Characterization of Cleveland Bay Horse Breed. Diversity 2019, 11, 174. https://doi.org/10.3390/d11100174
Khanshour AM, Hempsey EK, Juras R, Cothran EG. Genetic Characterization of Cleveland Bay Horse Breed. Diversity. 2019; 11(10):174. https://doi.org/10.3390/d11100174
Chicago/Turabian StyleKhanshour, Anas M., Eleanore K. Hempsey, Rytis Juras, and E. Gus Cothran. 2019. "Genetic Characterization of Cleveland Bay Horse Breed" Diversity 11, no. 10: 174. https://doi.org/10.3390/d11100174
APA StyleKhanshour, A. M., Hempsey, E. K., Juras, R., & Cothran, E. G. (2019). Genetic Characterization of Cleveland Bay Horse Breed. Diversity, 11(10), 174. https://doi.org/10.3390/d11100174



