Lack of Behavioral and Chemical Interference Competition for Refuges among Native Treefrogs and Invasive Cuban Treefrogs (Osteopilus septentrionalis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Treefrog Collection and Maintenance
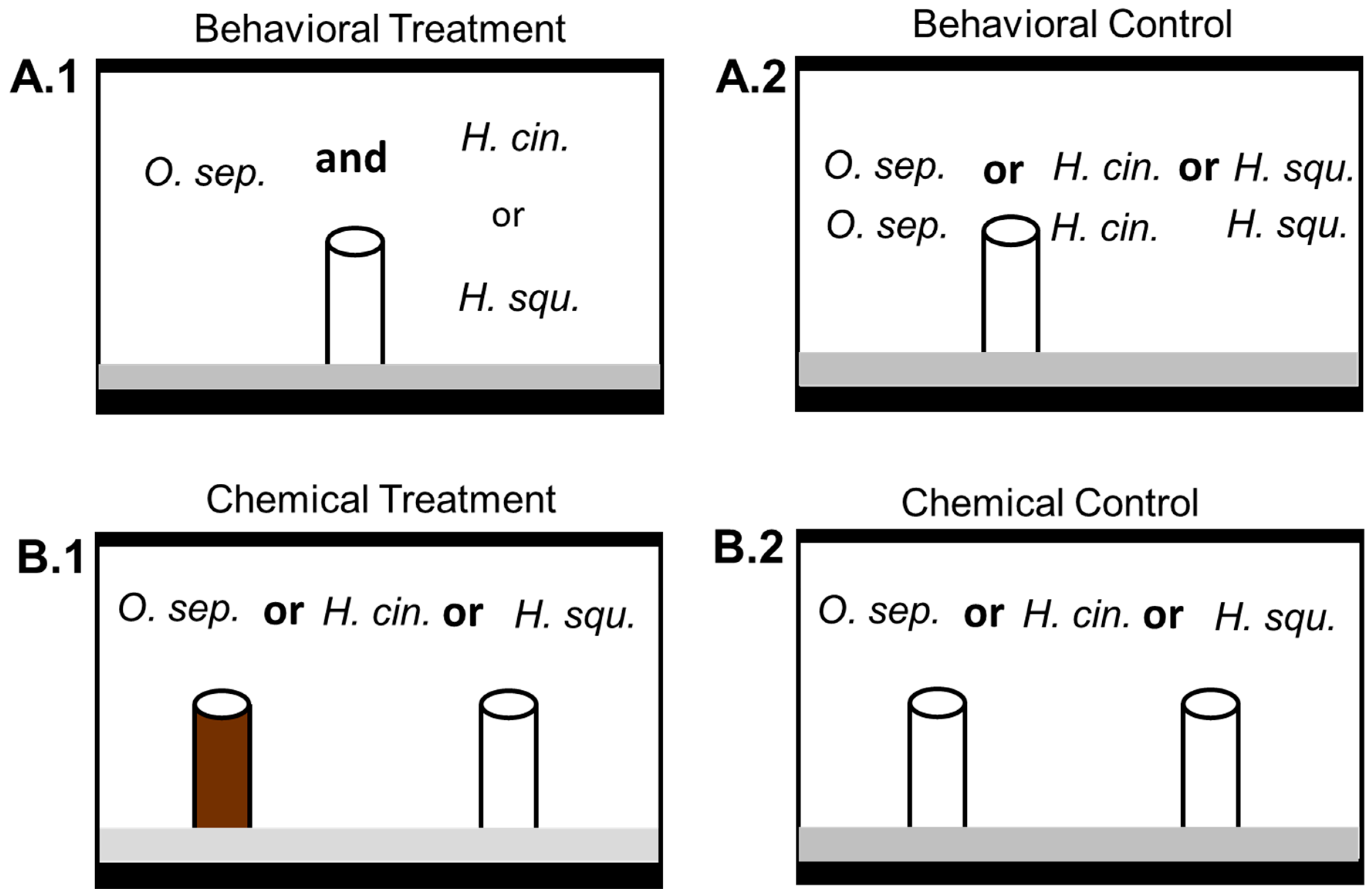
2.2. Behavioral Interference Trials
2.3. Chemical Interference Trials
3. Results
3.1. Behavioral Exclusion
3.2. Chemical Interference
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gause, G.F. The Struggle for Existence; Williams & Wilkins Company: Baltimore, MD, USA, 1934; p. 163. ISBN 0-486-49520-5. [Google Scholar]
- Jaeger, R.G.; Prosen, E.D.; Adams, D.C. Character displacement and aggression in two species of terrestrial salamanders. Copeia 2002, 2002, 391–401. [Google Scholar] [CrossRef]
- Pacala, S.W.; Roughgarden, J. Resource partitioning and interspecific competition in two two-species insular Anolis lizard communities. Science 1982, 217, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Cann, R.L.; Bodner, G.R. Incipient extinction of a major population of the Hawaii akepa owing to introduced species. Evol. Ecol. Res. 2008, 10, 931–965. [Google Scholar]
- Janssen, J.; Jude, D.J. Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, southern Lake Michigan, induced by the newly introduced round goby Neogobius melanostomus. J. Great Lakes Res. 2001, 27, 319–328. [Google Scholar] [CrossRef]
- Gallardo, B.; Clavero, M.; Sanchez, M.I.; Vila, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.S. Analyses of the Effects of an Exotic Lizard (Anolis sagrei) on a Native Lizard (Anolis carolinensis) in Florida, Using Islands as Experimental Units. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2000. [Google Scholar]
- D’Amore, A.; Kirby, E.; McNicholas, M. Invasive species shifts ontogenetic resource partitioning and microhabitat use of a threatened native amphibian. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 534–541. [Google Scholar] [CrossRef]
- Bohn, T.; Amundsen, P.A.; Sparrow, A. Competitive exclusion after invasion? Biol. Invasions 2008, 10, 359–368. [Google Scholar] [CrossRef]
- Meshaka, W.E., Jr. The Cuban Treefrog in Florida: Life History of a Successful Colonizing Species; University Press of Florida: Gainesville, FL, USA; p. 191. ISBN 0-8130-2109-X.
- Rice, K.G.; Waddle, J.H.; Miller, M.W.; Crockett, M.E.; Mazzotti, F.J.; Pervival, H.F. Recovery of native treefrogs after removal of nonindigenous Cuban treefrogs, Osteopilus septentrionalis. Herpetologica 2011, 67, 105–117. [Google Scholar] [CrossRef]
- Waddle, J.H.; Dorazio, R.M.; Walls, S.C.; Rice, K.G.; Beauchamp, J.; Schuman, M.J.; Mazzotti, F.J. A new parameterization for estimating co-occurrence of interacting species. Ecol. Appl. 2010, 20, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G. An exploratory assessment of Cuban treefrog (Osteopilus septentrionalis) tadpoles as predators of native and nonindigenous tadpoles in Florida. Amphib. Rept. 2005, 26, 571–575. [Google Scholar] [CrossRef]
- Knight, C.M.; Parris, M.J.; Gutzke, W.H.N. Influence of priority effects and pond location on invaded larval amphibian communities. Biol. Invasions 2009, 11, 1033–1044. [Google Scholar] [CrossRef]
- Duellmann, W.E.; Trueb, L. Biology of Amphibians; McGraw-Hill Book Company: New York, NY, USA, 1986; p. 670. ISBN 0-8018-4780-X. [Google Scholar]
- Lutz, B. Fighting and an incipient notion of territory in male tree frogs. Copeia 1960, 1960, 61–63. [Google Scholar] [CrossRef]
- Buchanan, B.W. Territoriality in the Squirrel Treefrog, Hyla squirella: Competition for Diurnal Retreat Sites. Master’s Thesis, University of Southwestern Louisiana, Lafayette, LA, USA, 1988. [Google Scholar]
- Stewart, M.M.; Rand, A.S. Vocalization and the defense of retreat sites by male and female frogs, Eleutherodactylus coqui. Copeia 1991, 1991, 1013–1024. [Google Scholar] [CrossRef]
- Wiewandt, T.A. Breeding biology of the Mexican leaf frog. Fauna 1971, 2, 29–34. [Google Scholar]
- Kiesecker, J.M.; Chivers, D.P.; Blaustein, A.R. The use of chemical cues in predator recognition by western toad tadpoles. Anim. Behav. 1996, 52, 1237–1245. [Google Scholar] [CrossRef]
- Stauffer, H.P.; Semlitsch, R.D. Effects of visual, chemical, and tactile cues of fish on the behavioural responses of tadpoles. Anim. Behav. 1993, 46, 355–364. [Google Scholar] [CrossRef]
- Chivers, D.P.; Kiesecker, J.M.; Wildy, E.L.; Blenden, L.K.; Kats, L.B.; Blaustein, A.R. Avoidance response of post-metamorphic anurans on cues of injured conspecifics and predators. J. Herpetol. 1999, 33, 472–476. [Google Scholar] [CrossRef]
- Chivers, D.P.; Wildy, E.L.; Kiesecker, J.M.; Blaustein, A.R. Avoidance response of juvenile Pacific treefrogs to chemical cues of introduced predatory bullfrogs. J. Chem. Ecol. 2001, 27, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.M.; Yeager, J.; Schulte, R.; Veith, M.; Werner, P.; Beck, L.A.; Lotters, S. The smell of success: Choice of larval rearing sites by means of chemical cues in a Peruvian poison frog. Animal Behav. 2011, 81, 1147–1154. [Google Scholar] [CrossRef]
- Johnson, S.A.; McGarrity, M.E.; Staudhammer, C.M. An effective chemical deterrent for invasive Cuban treefrogs. Hum. Wild. Interact. 2010, 4, 112–117. [Google Scholar]
- Moulton, C.A.; Flemming, W.J.; Nerney, B.R. The use of PVC pipes to capture hylid frogs. Herpetol. Rev. 1996, 27, 86–187. [Google Scholar]
- Boughton, R.G.; Staiger, J.; Franz, R. Use of PVC pipe refugia as a sampling technique or hylid treefrogs. Am. Mid. Nat. 2000, 144, 168–177. [Google Scholar] [CrossRef]
- Lever, C. Naturalized Reptiles and Amphibians of the World; Oxford University Press: Oxford, UK; p. 318. ISBN 978-0-19-850771-0.
- Barbour, T. Another introduced frog in North America. Copeia 1931, 1931, 140. [Google Scholar] [CrossRef]
- Krysko, K.L.; Enge, K.M.; Townsend, J.H.; Langan, E.M.; Johnson, S.A.; Campbell, T.S. New county records of amphibians and reptiles from Florida. Herpetol. Rev. 2005, 3, 85–87. [Google Scholar]
- McGarrity, M.E.; Johnson, S.A. Geographic trend in sexual size dimorphism and body size of Osteopilus septentrionalis (Cuban treefrog): Implications for invasion of the southeastern United States. Biol. Invasions 2009, 11, 1411–1420. [Google Scholar] [CrossRef]
- Beard, K.H.; Johnson, S.A.; Shiels, A.B. Frogs (coqui frogs, greenhouse frogs, Cuban tree frogs, and cane toads). In Ecology and Management of Terrestrial Vertebrate Invasive Species in the United States; Pitt, W.C., Beasly, J.C., Witmer, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 163–192. ISBN 978-1-4984-0482-3. [Google Scholar]
- Rödder, D.; Weinsheimer, F. Will future anthropogenic climate change increase the potential distribution of the alien invasive Cuban treefrog (Anura: Hylidae)? J. Nat. Hist. 2009, 43, 1207–1217. [Google Scholar] [CrossRef]
- Glorioso, B.M.; Waddle, J.H.; Muse, L.J.; Jennings, N.D.; Litton, M.; Hamilton, J.; Gergen, S.; Heckard, D. Establishment of the exotic invasive Cuban treefrog (Osteopilus septentrionalis) in Louisiana. Biol. Invasions 2018, 1–7. [Google Scholar] [CrossRef]
- Bartareau, T.M.; Meshaka, W.E. Osteopilus septentrionalis (Cuban treefrog). Diet. Herpetol. Rev. 2007, 38, 324–325. [Google Scholar]
- Glorioso, B.; Waddle, J.H.; Crockett, M.E.; Rice, K.G.; Precival, H.F. Diet of the invasive Cuban treefrog (Osteopilus septentrionalis) in pine rockland and mangrove habitats in South Florida. Caribb. J. Sci. 2012, 46, 346–355. [Google Scholar] [CrossRef]
- Hoffmann, K.E.; Johnson, S.A. Osteopilus septentrionalis (Cuban treefrog). Diet. Herpetol. Rev. 2008, 39, 339. [Google Scholar]
- Maskell, A.J.; Waddle, J.H.; Rice, K.G. Osteopilus septentrionalis. Diet. Herpetol. Rev. 2003, 34, 137. [Google Scholar]
- Wyatt, J.L.; Forys, E.A. Conservation implications of predation by Cuban treefrogs (Osteopilus septentrionalis) on native hylids in Florida. Southeast. Nat. 2004, 3, 695–700. [Google Scholar] [CrossRef]
- Love, W.B. Osteopilus septentrionalis (Cuban treefrog). Predation. Herpetol. Rev. 1995, 26, 201–202. [Google Scholar]
- Meshaka, W.E., Jr. Theft or cooperative foraging in the barred owl? Fla. Field Nat. 1996, 24, 15. [Google Scholar]
- Meshaka, W.E., Jr.; Ferster, B. Two species of snakes prey on Cuban treefrogs in southern Florida. Fla. Field Nat. 1995, 23, 97–98. [Google Scholar]
- Meshaka, W.E., Jr.; Jansen, K.P. Osteopilus septentrionalis (Cuban treefrog). Predation. Herpetol. Rev. 1997, 28, 147–148. [Google Scholar]
- Rodriguez, L.F. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol. Invasions 2006, 8, 927–939. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2017; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Forester, D.C.; Wisnieski, A. The significance of airborne olfactory cues to the recognition of home area by the dart-poison frog Dendrobates pumilio. J. Herpetol. 1991, 25, 502–504. [Google Scholar] [CrossRef]
- Waldman, B.; Bishop, P.J. Chemical communication in an archaic anuran amphibian. Behav. Ecol. 2004, 15, 88–93. [Google Scholar] [CrossRef]
- Flowers, M.A.; Graves, B.M. Juvenile toads avoid chemical cues from snake predators. Anim. Behav. 1997, 53, 641–646. [Google Scholar] [CrossRef]
- Pizzatto, L.; Child, T.; Shine, R. Why be diurnal? Shifts in activity time enable young cane toads to evade cannibalistic conspecifics. Behav. Ecol. 2008, 19, 990–997. [Google Scholar] [CrossRef]
- Chivers, D.P.; Smith, R.J.F. Fathead minnows, Pimephales promelas, acquire predator recognition when alarm substance is associated with the sight of unfamiliar fish. Anim. Behav. 1994, 48, 597–605. [Google Scholar] [CrossRef]
- Griffin, A.S.; Blumstein, D.T.; Evans, C.S. Training captive-bred or translocated animals to avoid predators. Cons. Biol. 2000, 14, 1317–1326. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs (Rana catesbeiana). Ecology 1997, 78, 1752–1760. [Google Scholar] [CrossRef]
- Savidge, J.A. Extinction of an island forest avifauna by and introduced snake. Ecology 1987, 68, 660–668. [Google Scholar] [CrossRef]
- Pearl, C.A.; Adams, M.J.; Schuytema, G.S.; Nebeker, A.V. Behavioral responses of anuran larvae to chemical cues of native and introduced predators in the pacific northwestern United States. J. Herpetol. 2003, 37, 572–576. [Google Scholar] [CrossRef]
- Hoffmann, K.E.; Johnson, S.J.; McGarrity, M.E. Interspecific variation in use of polyvinyl chloride (PVC) pipe refuges by hylid frogs: A potential source of capture bias. Herpetol. Rev. 2009, 40, 423–426. [Google Scholar]
- Campbell, T.S.; Irvin, P.; Campbell, K.R.; Hoffmann, K.; Dykes, M.E.; Harding, A.J.; Johnson, S.A. Evaluation of a new technique for marking anurans. Appl. Herpetol. 2009, 6, 247–256. [Google Scholar] [CrossRef]
| Treefrog Pairing | Neither | A Only | B Only | Both | Behavioral Interference on Species A | |||
|---|---|---|---|---|---|---|---|---|
| (A) | (B) | N | (00) | (A0) | (0B) | (AB) | LR | p |
| Squirrel | Cuban | 30 | 8 | 12 | 4 | 6 | 1.69 | 0.19 |
| Squirrel | 30 | 7 | 2 | 9 | 12 | --- | --- | |
| Green | Cuban | 30 | 13 | 4 | 7 | 6 | 0.13 | 0.72 |
| Green | 30 | 17 | 5 | 3 | 5 | --- | --- | |
| Squirrel | 30 | 8 | 4 | 12 | 6 | 2.89 | 0.09 | |
| Cuban | Green | 30 | 13 | 7 | 4 | 6 | 2.13 | 0.14 |
| Cuban | 30 | 11 | 3 | 13 | 3 | --- | --- | |
| Treefrog Species | Experimental (Observed) | Controls (Expected) | |||||
|---|---|---|---|---|---|---|---|
| Residue PVC | Clean PVC | Neither | Clean PVC A | Clean PVC B | Neither | p | |
| Cuban | 3 | 11 | 16 | 4 | 7 | 19 | 0.65 |
| Squirrel | 11 | 9 | 10 | 9 | 13 | 8 | 0.68 |
| Green | 5 | 9 | 16 | 11 | 5 | 14 | 0.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, K.E.; McGarrity, M.E.; Johnson, S.A. Lack of Behavioral and Chemical Interference Competition for Refuges among Native Treefrogs and Invasive Cuban Treefrogs (Osteopilus septentrionalis). Diversity 2018, 10, 78. https://doi.org/10.3390/d10030078
Hoffmann KE, McGarrity ME, Johnson SA. Lack of Behavioral and Chemical Interference Competition for Refuges among Native Treefrogs and Invasive Cuban Treefrogs (Osteopilus septentrionalis). Diversity. 2018; 10(3):78. https://doi.org/10.3390/d10030078
Chicago/Turabian StyleHoffmann, Kristine E., Monica E. McGarrity, and Steve A. Johnson. 2018. "Lack of Behavioral and Chemical Interference Competition for Refuges among Native Treefrogs and Invasive Cuban Treefrogs (Osteopilus septentrionalis)" Diversity 10, no. 3: 78. https://doi.org/10.3390/d10030078
APA StyleHoffmann, K. E., McGarrity, M. E., & Johnson, S. A. (2018). Lack of Behavioral and Chemical Interference Competition for Refuges among Native Treefrogs and Invasive Cuban Treefrogs (Osteopilus septentrionalis). Diversity, 10(3), 78. https://doi.org/10.3390/d10030078






