Molecular and Morphological Phylogenetic Analyses of New World Cycad Beetles: What They Reveal about Cycad Evolution in the New World
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Analysis
2.2. DNA Analysis
3. Results and Discussion
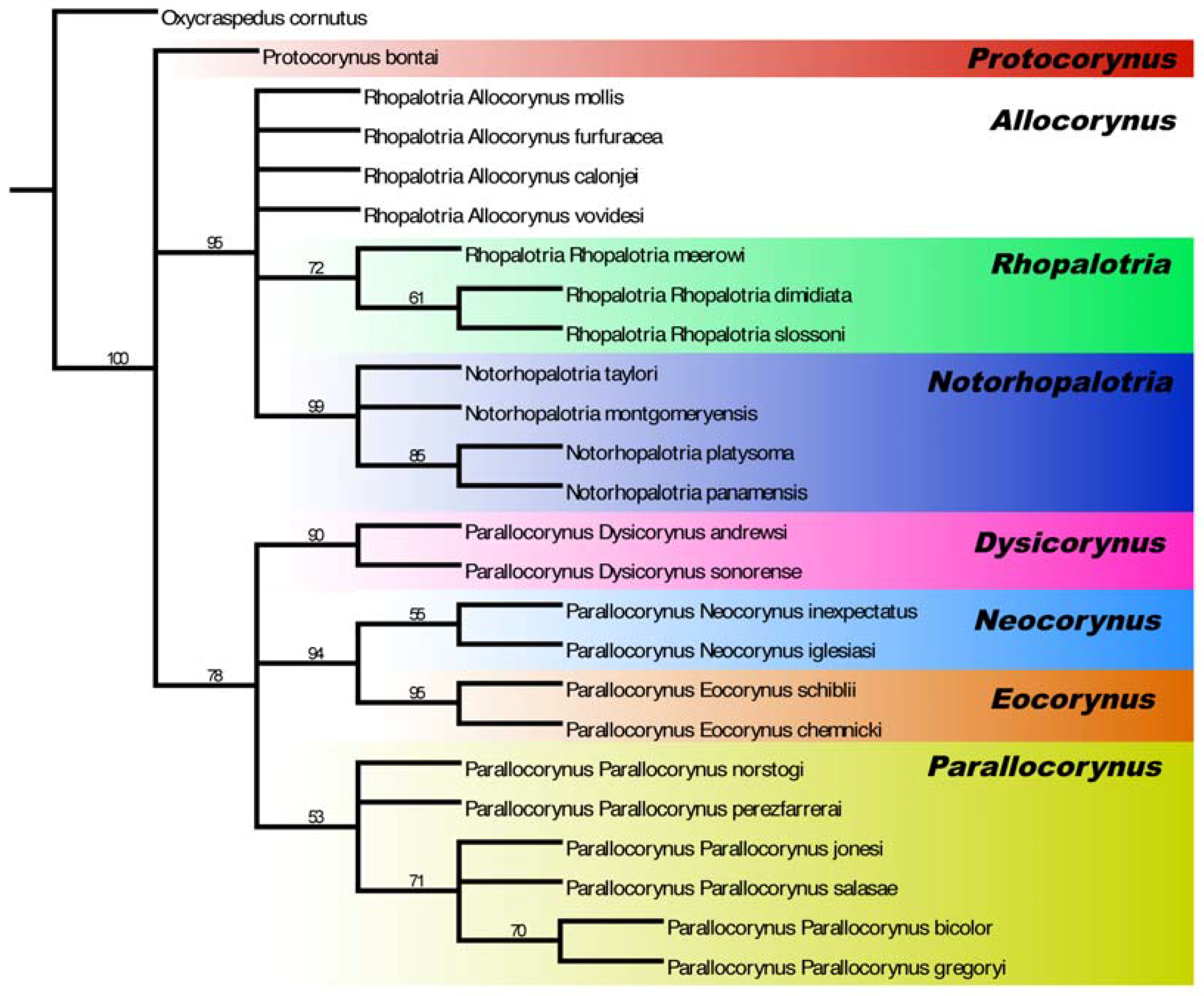
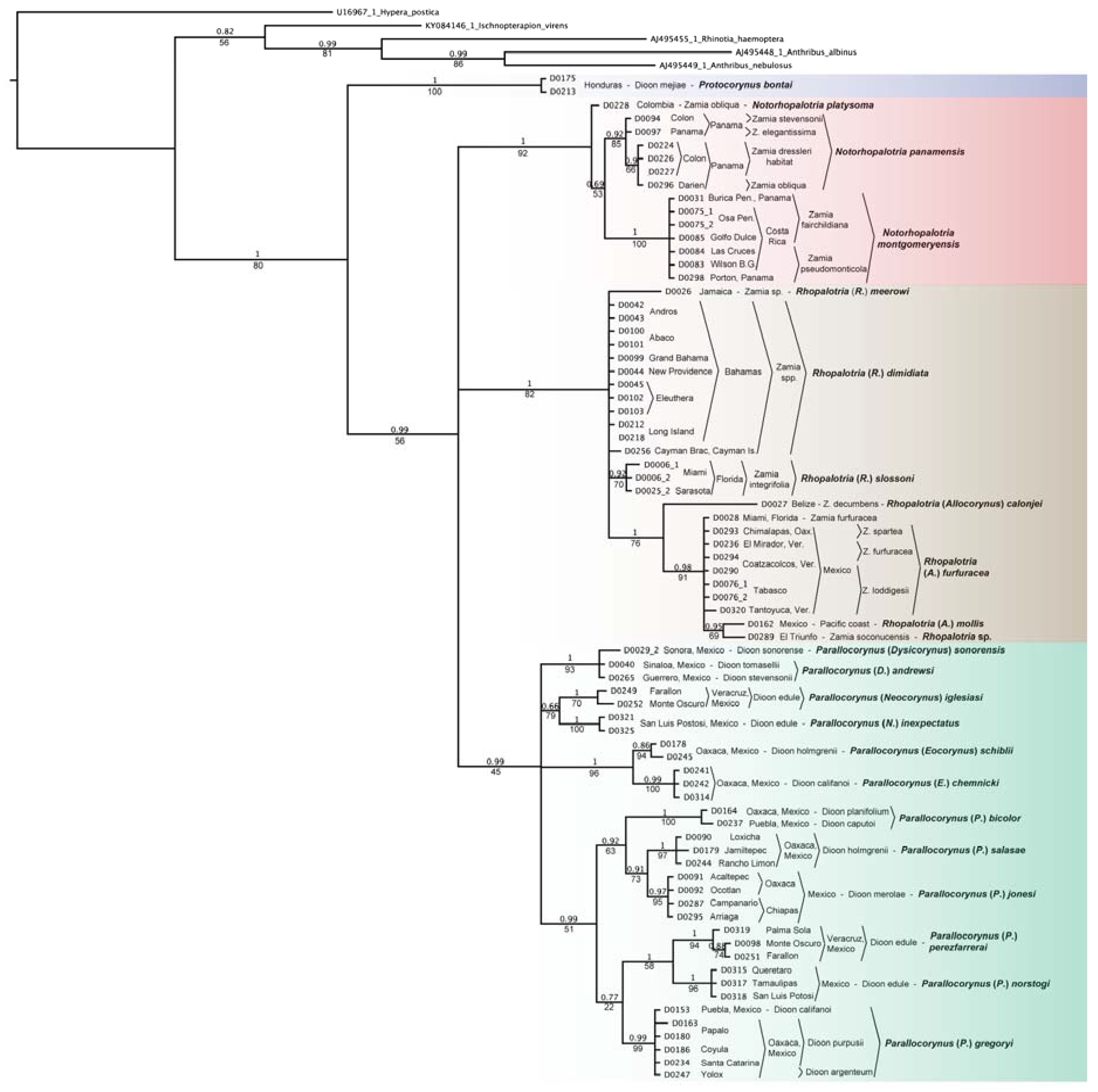
3.1. Allocorynina Trees
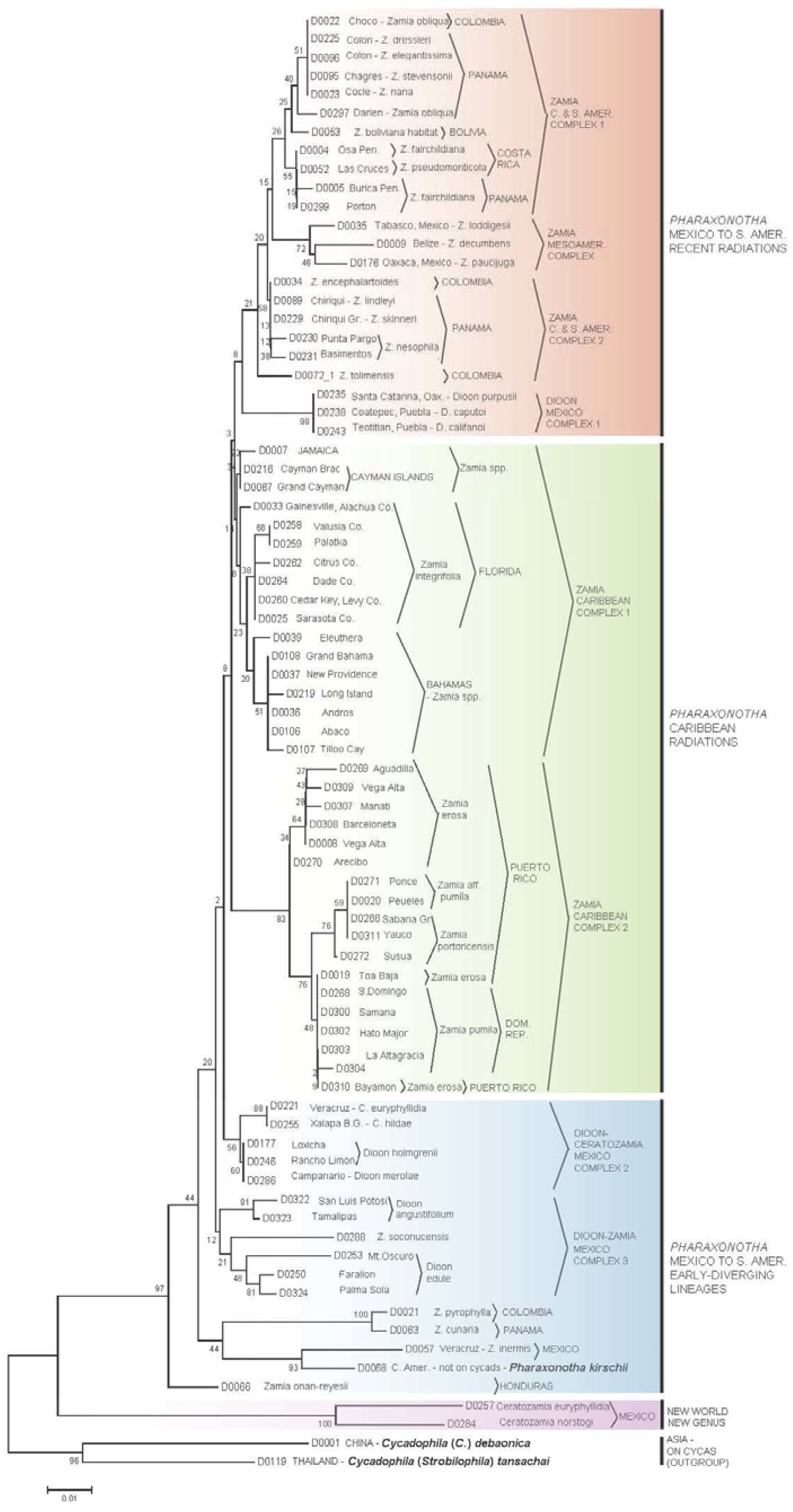
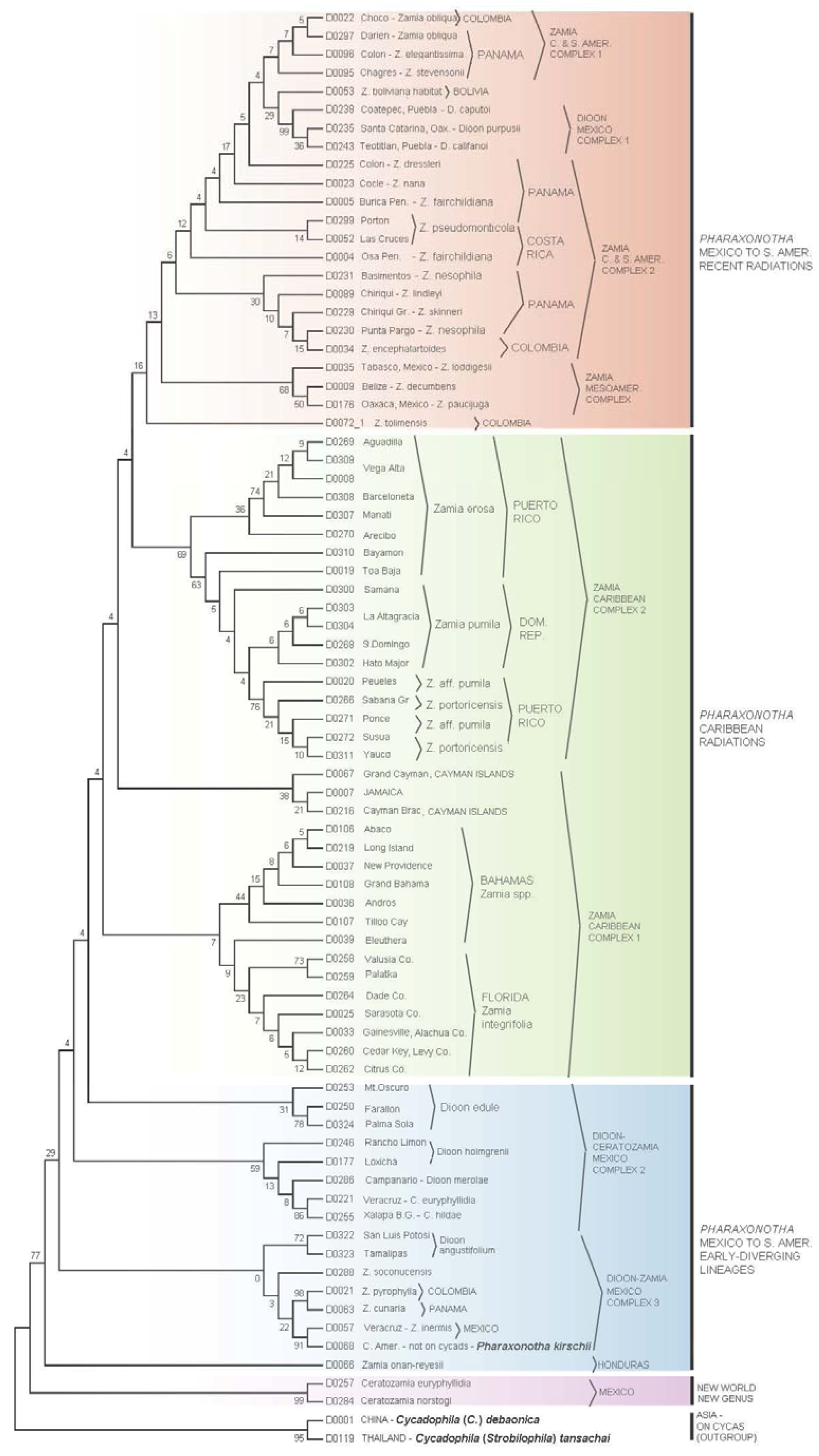
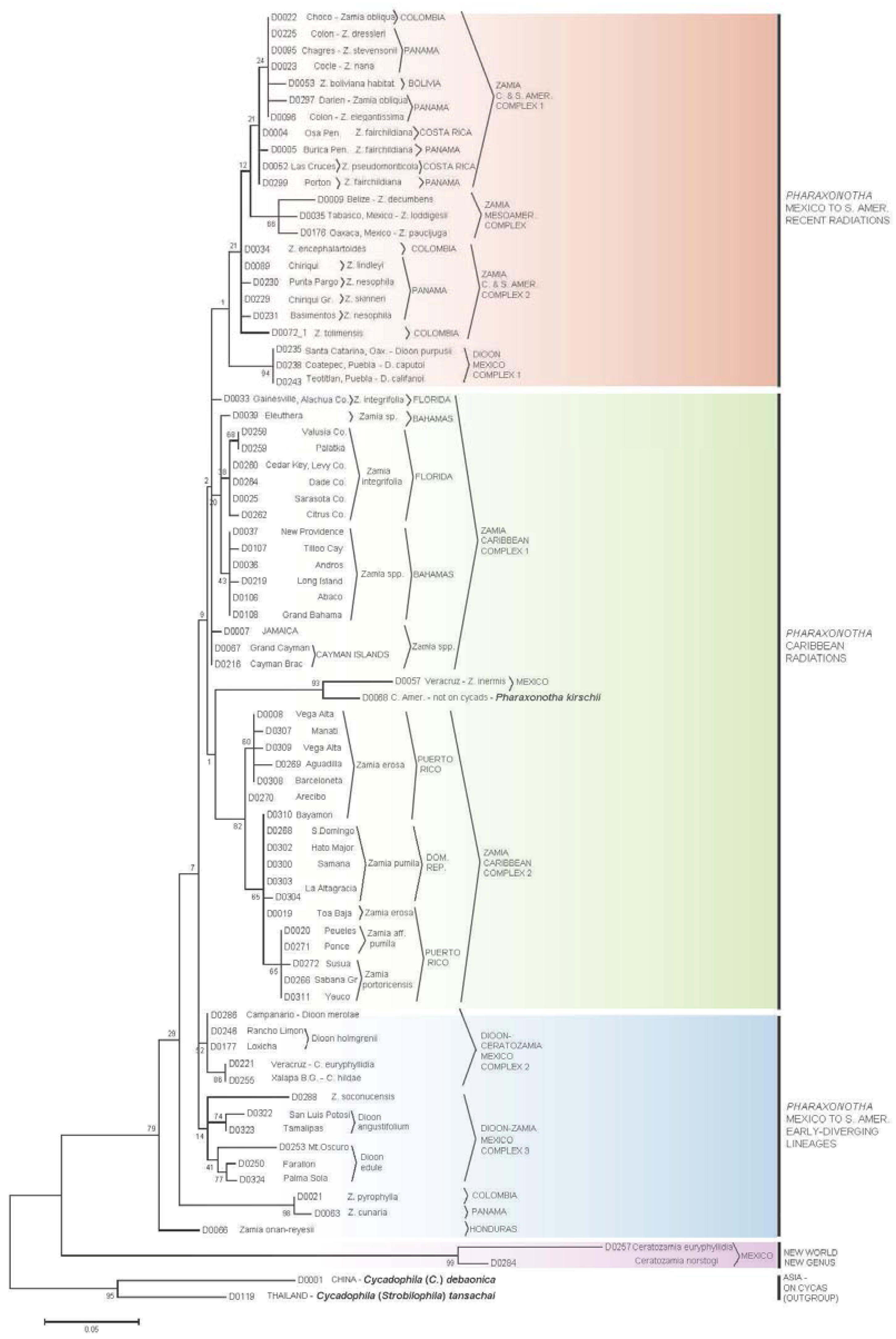
3.2. Erotylidae Tree
3.3. Implications for the Evolution of Cycad Hosts
3.3.1. Using Beetle Trees to Generate Hypotheses of Cycad Evolution
3.3.2. Cycad Hypotheses Based on the Allocorynina Trees
- (1)
- The presence of one or two species of Allocorynina in all species of Dioon sampled compared with its absence from many Zamia species and its complete absence in the other New World cycad genera Ceratozamia and Microcycas suggests that Dioon is the earliest host lineage colonized by Allocorynina weevils, with one or possibly two host-shifts onto Zamia. In this hypothesis, Allocorynina are the original pollinators in the genus Dioon, while erotylid beetles are later colonists in Dioon.
- (2)
- Based on the morphological and genetic analyses of its pollinator Dioon mejiae, located on the Chortis block, a tectonic terrane roughly corresponding to the country of Honduras [58], is hypothesized to be one of the earliest bifurcating lineages within Dioon.
- (3)
- Species of narrow-leaflet Dioon in Mexico form four lineages with distinct biogeographic distributions: (A) western Mexico lineage along the Pacific drainage of the Sierra Madre Occidental from Sonora to Guerrero consisting of D. sonorense (De Luca, Sabato and Vázq.Torres) Chemnick, T.J.Greg. and Salas-Mor., D. stevensonii Nic.-Mor. and Vovides and D. tomasellii De Luca, Sabato and Vázq.Torres; (B) eastern Mexico lineage along the Sierra Madre Oriental from Nuevo Leon to Veracruz consisting of D. angustifolium Miq. and D. edule Lindl.; (C) south central Mexico lineage consisting of D. argentium, D. califanoi, and D. purpusii; (D) southern Mexico lineage along the Pacific drainage of Oaxaca to Chiapas, consisting of D. caputoi De Luca, Sabato and Vázq.Torres, D. holmgrenii De Luca, Sabato and Vázq.Torres, D. merolae De Luca, Sabato and Vázq.Torres and D. planifolium Salas-Mor., Chemnick and T. J. Greg.
- (4)
- For the eastern Mexico lineage (group 3B above) Dioon edule (including D. angustifolium) north of the Trans-Mexican Volcanic Belt in the states of Nuevo Leon, Queretaro, San Luis Potosi, and Tamaulipas is likely a distinct species from D. edule south of this mountain range in Veracruz; the two lineages of Allocorynina and the lineage of erotylids inhabiting this cycad species group support this division.
- (5)
- The absence of Allocorynina in the periphery of the geographic range of Zamia (e.g., eastern part of the Greater Antilles and much of Panama and South America) suggests that colonization of Zamia by Allocorynina is relatively recent and perhaps an ongoing ecological and evolutionary process. The alternate hypothesis is that there may have been a more widespread distribution on Zamia, but these weevils have suffered selective extinction in parts of their range.
- (6)
- The shift of Allocorynina from Dioon onto Zamia may have occurred during major tectonic events in the formation of Central America when landmasses were moving through the region, emerging from the sea, and/or colliding with Mesoamerica [59,60]; during this time, lineages of cycads (including Zamia and/or possibly other extinct cycad lineages) and the Allocorynina associated with them migrated in three directions: (A) south into Central America, (B) east into the Caribbean islands, and (C) within Mesoamerica.
- (7)
- Zamia obliqua A. Braun in the Choco of Colombia is likely a different species from Z. obliqua in the Darien of Panama based on genetic differences in the Allocorynina inhabiting their respective cones.
3.3.3. Cycad Hypotheses Based on the Erotylidae Tree
- (I)
- The most early-diverging lineage of cycad-associated erotylid pharaxonothine beetles in the New World is confined to the genus Ceratozamia. This branching pattern is consistent with fossil evidence indicating that the Ceratozamia lineage may have first evolved in Europe in the mid Cenozoic and then migrated to North America prior to the complete separation of these continents [61]. In addition, the apparent close relation between cycad-associated erotylids of the New World with those found on Cycas in Asia, suggest that these beetles may have an ancient Laurasian association with cycads that predates the breakup of Laurasia.
- (II)
- Two early-diverging erotylid beetle lineages associated with Zamia are located in: (a) Honduras on Z. onan-reyesii and (b) The northern South America-Darien region on Zamia cunaria and Z. pyrophylla. We may hypothesize that these host lineages of Zamia are among the earliest to diverge for the genus and are likely relicts from an earlier radiation of Zamia throughout these regions.
- (III)
- The presence of erotylid beetles in the cones of all species of Zamia that have been carefully sampled suggests that these were the original pollinators of Zamia. In this hypothesis, the Allocorynina weevils are later colonists of Zamia.
- (IV)
- In addition to old relictual clades in hypothesis II, the existence of three separate derived clades of Pharaxonotha beetles on Zamia suggests that at least three recent and separate radiations of Zamia have occurred in the following regions: (a) A radiation into the eastern islands of the Greater Antilles, which includes Hispaniola and Puerto Rico, probably beginning when these landmasses were more closely associated with Central America [60]; (b) A more recent radiation into the western islands of the Greater Antilles, including Cuba, Cayman Islands and Jamaica and neighboring landmasses of the Bahamas and Florida; and (c) Sister to these two Caribbean lineages, a recent radiation onto Zamia in Mesoamerica, Central America, and northern South America.
- (V)
- The separation of Pharaxonotha beetles, that inhabit Dioon cones, into three distinct and not closely related clades and the absence of erotylids on one species, D. mejiae in Honduras, at the periphery of the range of Dioon, suggests that erotylids colonized Dioon from the Zamia lineage in three separate host shift events and that these host shifts have occurred relatively recently compared to the radiation of Allocorynina in Dioon.
- (VI)
- At least one recent host-shift of Pharaxonotha, originating from Zamia, have occurred onto Ceratozamia. A larger and wider sampling of Ceratozamia beetles may reveal more than one host shift. These coexist within Ceratozamia cones with the more ancient erotylids beetles discussed in hypothesis I, so that now two disparate lineages of Pharaxonothinae coinhabit Ceratozamia cones. This host-shift radiation is allied with those in Dioon, suggesting important watershed periods in cycad evolution when exchange of pollinators occurred among cycad genera in the New World. Deeper study of these periods may be crucial in understanding the relatively recent resurgence of cycad evolution that have been proposed [62,63,64].
- (VII)
- The pattern of population genetic variation of Pharaxonotha beetles that presumably pollinate Zamia in Puerto Rico and Hispaniola mirrors to a great extent the population genetic variation exhibited by the Zamia on those islands [65]. These mirroring patterns suggest that the mobility and/or abundance of a cycad’s pollinator may influence gene flow in its host cycad and consequently the speciation pattern of its host. For example, observations [66] suggests that unlike other cycad beetles, this lineage of Pharaxonotha is highly sensitive to human disturbance of its vegetative habitat and easily becomes rare or locally extinct as a result. This susceptibility to disturbance and the low ability to recolonize its host from nearby populations suggests low mobility and low ability to mediate gene flow in its host Zamia. The resulting effect is to produce local reproductive isolation of cycad populations that appear on casual observation to have continuous distributions.
- (VIII)
- Based on population genetic variation of beetles discussed in hypothesis VII, the Zamia populations near Bayamon and Toa Baja, Puerto Rico may be conspecific with Zamia pumila in Hispaniola; furthermore, Z. pumila populations in Hispaniola may be recent colonists from an ocean dispersal event originating from Bayamon and Toa Baja.
3.3.4. Independent Tests of Beetle Generated Hypotheses
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
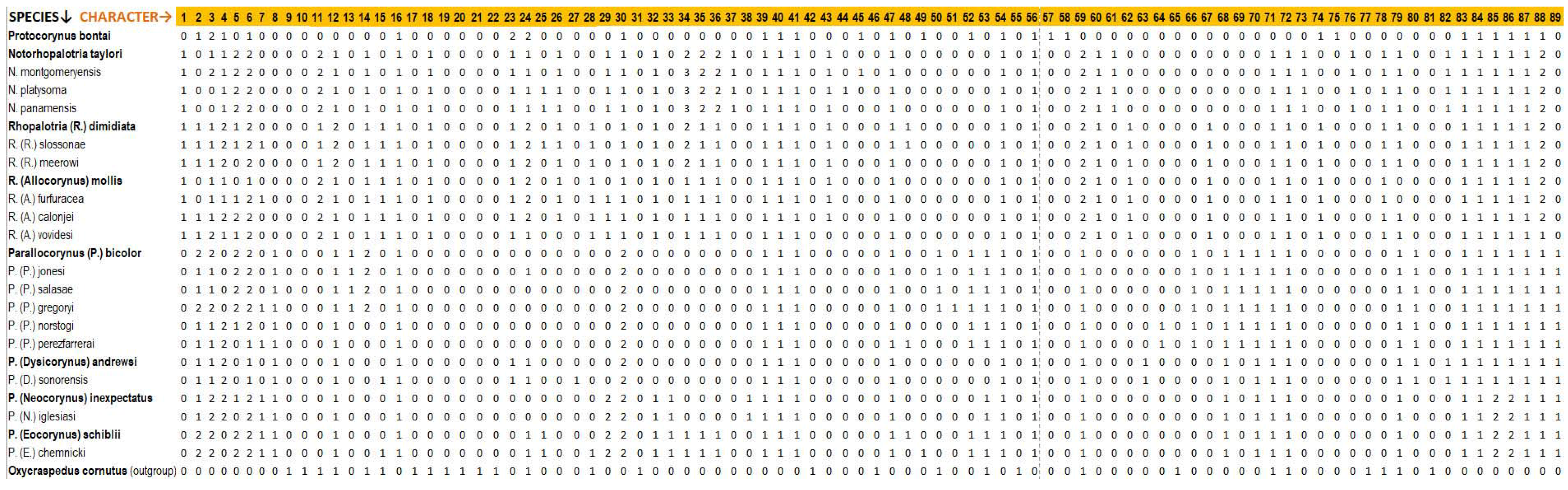
Appendix A
- (1)
- Labial palp: (0) 3 segments; (1) 2 segments. The presence of 2-segmented labial palps is a synapomorphy for the Notorhopalotria-Rhopalotria clade.
- (2)
- Mean male rostral length/pronotal length (RL/PL): (0) <1.0; (1) >1.0, and <1.30; (2) >1.30. High male RL/PL are characteristic of Parallocorynus subgenus Eocorynus and the subgenus Parallocorynus bicolor-jonesi-salasae-gregoryi clade, while low male RL/PL (rostral length < pronotal length) is found in the genus Notorhopalotria and two species of Rhopalotria subgenus Allocorynus.
- (3)
- Mean female RL/PL: (0) <1.25; (1) >1.25, and <1.50; (2) >1.50. High female RL/PL are characteristic of Parallocorynus subgenera Eocorynus and Neocorynus, but has arisen independently in females of other taxa including Protocorynus bontai, Notorhopalotria montgomeryensis, Rhopalotria vovidesi, Parallocorynus (Parallocorynus) bicolor, and P. (P.) gregoryi.
- (4)
- Interocular width/head width at eye: (0) = or >0.5; (1) = or <0.4; (2) >0.4, and <0.5. Short interocular widths are indicative of large eyes and are found in the genera Protocorynus and Notorhopalotria and in Rhopalotria in the mollis-furfuracea species group; long interocular distances are indicative of smaller eyes and are characteristic of Parallocorynus in the subgenus Eocorynus and the subgenus Parallocorynus bicolor-jonesi-salasae-gregoryi clade.
- (5)
- Male: mean post-ocular head width/head width at eye (POW/HW): (0) = or <0.95; (1) >0.95 and = or <1.0; (2) >1.0. Wide male post-ocular head width is characteristic of Notorhopalotria, Parallocorynus subgenus Eocorynus, the subgenus Parallocorynus P. bicolor-jonesi-salasae-gregoryi clade, and Rhopalotria calonjei.
- (6)
- Female: mean POW/HW: (0) = or < 0.95; (1) >0.95 and = or < 1.0; (2) >1.0. Medium to short female postocular head widths are characteristic of the Allocorynina compared with the narrow width in the outgroup genus Oxycraspedus.
- (7)
- POW/HW: sexually dimorphic (no overlap): (0) No (dimorphism absent); (1) Yes (dimorphism present). Strong sexual dimorphism in post-ocular head width is characteristic in Parallocorynus subgenus Eocorynus, but has arisen six separate times in other genera and subgenera.
- (8)
- Transverse postocular groove: (0) Absent; (1) Present. Character 1 is a synapomorphy for the genus Parallocorynus.
- (9)
- Antennal insertion shape: (0) Foveiform to slightly oval; (1) Sulciform.
- (10)
- Antennal club, connection of antennomeres: (0) Distinct, 9–10 and 10–11 loosely connected; (1) Distinct, 9–10 loosely connected, 10–11 tightly joined.
- (11)
- Number of pockets on each side club antennomeres: (0) 1; (1) 2; (2) 3. One pocket is characteristic for Parallocorynus and Protocorynus; two pockets is characteristic of Rhopalotria subgenus Rhopalotria.
- (12)
- Antennal club pocket shape: (0) Half circle (autapomorphy for Protocorynus); (1) Oval to round; (2) Elongate oval with irregular outline. Character state 2 is a synapomorphy for Rhopalotria subgenus Rhopalotria.
- (13)
- Funiclular antennomere 1 in females: (0) Approximately symmetrical; (1) Strongly asymmetrical. Synapomorphy for the Parallocorynus bicolor-jonesi-salasae-gregoryi clade.
- (14)
- Mean scape length in males: (0) >1.1X and <1.8X eye length; (1) <1.1X eye length; (2) >1.8X eye length. Scape length shorter than 1.1X eye length in males is characteristic for Notorhopalotria and Rhopalotria. Scape length relative to eye length is also a synapomorphy for the Parallocorynus bicolor-jonesi-salasae-gregoryi clade; this is the only clade where the scape length routinely exceeds 2X eye length.
- (15)
- Mean scape length in males: (0) <1.27X length of funicular antennomeres 1 and 2; (1) >1.27X length of funicular antennomeres 1 and 2. Scape length shorter than 1.27X length of funicular antennomeres 1 and 2 in males is characteristic for Rhopalotria.
- (16)
- Gular suture: (0) Entirely separated; (1) Fused.
- (17)
- Sulcus at posterior margin of eye: (0) Absent; (1) Present and extending around dorsal margin of eye.
- (18)
- Collar on anterior pronotal margin: (0) Present; (1) Absent. Character shared between Protocorynus and Parallocorynus.
- (19)
- Pronotal apex: (0) Without constriction; (1) With constriction.
- (20)
- Lateral margin of pronotum: (0) Not carinate; (1) With carinae.
- (21)
- Lateral pronotal margin: (0) Not crenulate; (1) Crenulate.
- (22)
- Shape of prothorax: (0) Anterior lateral angles not produced; (1) Anterior lateral angles produced forward.
- (23)
- Male: mean pronotal width/pronotal length (PW/PL): (0) < or = 1.35; (1) >1.35 and = or < 1.5; (2) >1.5. Within the Allocorynina a relatively narrow pronotum in males is characteristic for Parallocorynus except for an inferred reversal in the subgenus Dysicorynus.
- (24)
- Female: mean PW/PL: (0) <1.25; (1) >1.25 and <1.45; (2) >1.45. Within the Allocorynina, a relatively wide pronotum is characteristic in Protocorynus and Rhopalotria (except R. vovidesi).
- (25)
- PW/PL: (0) Overlap between sexes; (1) No overlap between sexes. Strong sexual dimophism in this character is characteristic of Parallocorynus subgenus Eocorynus, but has arisen independently twice in Notorhopalotria and Rhopalotria.
- (26)
- Anterior pronotal setal fringe: (0) Present; (1) Obsolete between eyes (not protruding beyond margin). Shared character between Notorhopalotria and Rhopalotria (except for R. vovidesi).
- (27)
- Fovea on pronotum: (0) Absent; (1) Present.
- (28)
- Notopleural suture reaching anterior margin of pronotum: (0) Yes; (1) No. Characteristic for Rhopalotria and with a reversal for Parallocorynus chemnicki.
- (29)
- Mean distance from procoxa to anterior margin of prosternum/distance from procoxa to posterior margin of prosternum: (0) >2.2 and <3.8; (1) <2.2; (2) >3.8. High ratios indicate that the procoxae are inserted on the posterior side of the prosternum and is a synapomorphy for Parallocorynus subgenera Eocorynus and Neocorynus.
- (30)
- Procoxae separated by: (0) Broad sclerite; (1) Sclerotized septum; (2) Not separated. The lack of septum is a synapomophy for Parallocorynus.
- (31)
- Forecoxae: (0) Partially open laterally; (1) Completely closed laterally.
- (32)
- Male profemur: (0) Not enlarged; (1) Enlarged. Enlarged profemora in males appears to have arisen independently in Notorhopalotria, Rhopalotria, and the Eocorynus-Neocorynus clades.
- (33)
- Male profemur granular field: (0) Absent (1) Present. Presence is a synapomorphy for the Eocorynus-Neocorynus clade.
- (34)
- Male profemoral ventrodistal spine number: (0) Absent; (1) One; (2) Two; (3) More than two. Profemoral spines in males appear to have arisen three times independently in the Notorhopalotria, Rhopalotria, and the Eocorynus clades.
- (35)
- Male profemoral spine position at ventrodistal pit: (0) Absent; (1) At proximal apex; (2) Lateral.
- (36)
- Male profemoral spine location from margin of ventrodistal pit: (0) Absent; (1) At margin; (2) Away from margin. Spine location away from pit is a synapomorphy for Notorhopalotria.
- (37)
- Male profemur with a longitudinal ventroproximal ridge: (0) Absent; (1) Present. Synapomorphy for Notorhopalotria.
- (38)
- Male: profemora with ventrodistal angulation: (0) Absent; (1) Present. Synapomorphy for subgenus Neocorynus.
- (39)
- Meso- and metafemora: (0) Not conspicuously compressed; (1) Strongly compressed.
- (40)
- Meso- and metafemora: (0) Without dorsal crenulation; (1) With dorsal crenulation.
- (41)
- Tibial spurs: (0) Present and articulated; (1) Present but fused.
- (42)
- Basal tarsal segment: (0) Subequal to second segment; (1) Much shorter than second and almost concealed.
- (43)
- Pronotum, frons, and dorsal surfaces of profemora with fine reticulation: (0) No; (1) Yes. Synapomorphy for Notorhopalotria-Rhopalotria clade (except for a reversal in R. vovidesi).
- (44)
- Pronotum compressed: height < 0.4X width: (0) No; (1) Yes.
- (45)
- Pronotum consistently bicolored: (0) No; (1) Yes. Characteristic of Protocorynus, with one independent reversal in N. montgomeryensis.
- (46)
- Punctures on elytra: (0) Irregularly distributed; (1) Ordered longitudinally but not in perfect striae.
- (47)
- Elytra: (0) With wing locking mechanism, closing to apices, concealing pygidium; (1) Without wing locking mechanism, rounded at apex, pygidium visible.
- (48)
- Elytra bicolored: (0) No; (1) Yes. This character has arisen independently three times within Allocorynina.
- (49)
- Color of frons black (vs. brown): (0) No; (1) Yes. This character has arisen twice in Allocorynina in Protocorynus and Parallocorynus chemnicki.
- (50)
- Metasternum color black (vs. brown): (0) No; (1) Yes. Within the Allocorynina, this character has arisen once in the Parallocorynus subgenus Parallocorynus bicolor-jonesi-salasae-gregoryi clade.
- (51)
- Meso- and metafemur always black: (0) No; (1) Yes.
- (52)
- Tibia and femur colors often differ: (0) No; (1) Yes. This character is found in Parallocorynus in the subgenus Eocorynus, and in the subgenus Parallocorynus bicolor-jonesi-salasae-gregoryi clade.
- (53)
- Rostrum color: (0) Brown; (1) Black. A black rostrum has arisen independently twice in Parallocorynus.
- (54)
- Mesoventrite: (0) Flat with intercoxal process strongly projected ~45° angle; (1) Slightly proclinate with intercoxal process on same level.
- (55)
- Metaventrite: (0) Convex; (1) Disk flattened.
- (56)
- Metaventrite, latero-posteriorly: (0) Gently rounded; (1) Sharply declined.
- (57)
- Wing vein rm: (0) Not sclerotized (obsolete); (1) Sclerotized.
- (58)
- Wing vein Mr spur: (0) Present; (1) Absent.
- (59)
- Wing vein 1A2 length: (0) >1A1; (1) <1A1; (2) Missing. Missing vein is a synapomorphy for the Notorhopalotria and Rhopalotria clade.
- (60)
- Wing vein 1A1: (0) Present; (1) Missing. Missing 1A1 vein is a synapomorphy for the Notorhopalotria and Rhopalotria clade.
- (61)
- Wing vein 3A: (0) Extends beyond confluence with 2A; (1) Obsolete beyond confluence with 2A. Character state 1 is a synapomorphy for the Notorhopalotria.
- (62)
- Aedeagus apex subtruncate: (0) No; (1) Yes. Synapomorphy for Rhopalotria.
- (63)
- Aedeagus apex length: (0) Approximately equal to own width; (1) Twice own width. Character state 1 is a synapomorphy for Parallocorynus subgenus Dysicorynus.
- (64)
- Gonopore with sclerotized knob: (0) No; (1) Yes. Synapomorphy for the Parallocorynus norstogi-perezfarrerai clade.
- (65)
- Gonopore position: (0) Dorsal; (1) Ventrolateral.
- (66)
- Aedeagus internal sac with ventral strut: (0) No; (1) Yes. Synapomorphy for the Parallocorynus subgenus Parallocorynus.
- (67)
- Aedeagus internal sac with transfer apparatus: (0) No; (1) Yes. Synapomorphy for Rhopalotria.
- (68)
- Aedeagus internal sac w/dart: (0) No; (1) Yes. Synapomorphy for Parallocorynus.
- (69)
- Aedeagus internal sac with dorsal pleats: (0) Absent; (1) Present. Synapomorphy for Parallocorynus subgenus Parallocorynus.
- (70)
- Aedeagus with prominent sclerotized transverse bridge: (0) No; (1) Yes. Synapomorphy for Parallocorynus.
- (71)
- Aedeagus shape: (0) Trough-shaped; (1) Flattened.
- (72)
- Tegmen dorsal bridge length from base to its junction with the apical plate extends <1/2 length of apical plate (vs. greater than): (0) No; (1) Yes.
- (73)
- Tegmen apical setae: (0) Absent or length < width of apical plate; (1) Length > width of apical plate. Character state 1 is a synapomorphy for Notorhopalotria.
- (74)
- Tegmen apical visor: (0) Absent; (1) Present. Synapomorphy for Rhopalotria, with the character arising independently in Protocorynus where the visor extends across lateral and part of ventral margin.
- (75)
- Tegmen apical visor curled laterally: (0) No; (1) Yes.
- (76)
- Tegmen apical plate curls transversely: (0) No; (1) Yes. Synapomorphy for Notorhopalotria.
- (77)
- Tegmen apodeme height: (0) <width of apical plate; (1) >width of apical plate.
- (78)
- Female: sternum VIII distal half of arms: (0) Strongly converge; (1) Mostly parallel. Character state 1 is a synapomorphy for the Notorhopalotria and Rhopalotria clade.
- (79)
- Female: sternum VIII arm length: (0) About equal to length of apodeme; (1) >1.5 length of apodeme. Character state (0) is found only in the R. furfuracea-R. mollis clade and has arisen independently in Protocorynus.
- (80)
- Female: sternum VIII arms: (0) Curved evenly; (1) With sharp angulate bend. Angulate bends is characteristic of Parallocorynus with a reversal in the subgenera Eocorynus and Neocorynus.
- (81)
- Female sternum VIII: junction of arms: (0) Diverging at angle <90°; (1) Forming transverse bar.
- (82)
- Female: spermathecal tube length: (0) <sternum VIII length; (1) >sternum VIII length. Long tube is characteristic of Notorhopalotria and Parallocorynus subgenus Dysicorynus.
- (83)
- Spermatheca: (0) Present and falciform; (1) Absent.
- (84)
- Spermathecal gland: (0) Tapering to spermathecal duct; (1) Forming common tube with duct.
- (85)
- Larval feeding site: (0) Female cone; (1) Male sporophyll; (2) Male cone axis.
- (86)
- Pupation site: (0) Female cone; (1) Male cone; (2) Outside of cone. Pupation site outside of cone is a synapomorphy for the Eocorynus-Neocorynus clade.
- (87)
- Host plant family: (0) Araucariaceae; (1) Zamiaceae. Synapomorphy for Allocorynina.
- (88)
- Host genus: (0) Araucaria; (1) Dioon; (2) Zamia.
- (89)
- Adult gut contents: (0) Mainly cone tissues other than pollen; (1) Predominately pollen. Character state 1 is a synapomorphy for Parallocorynus.
Appendix B
Appendix C
- (1)
- Male profemur with a ventroproximal ridge.
- (2)
- Male profemoral spine(s) located distantly from margin of profemoral apical pit.
- (3)
- Wing veins 1A1, 1A2, and 3A obsolete and not reaching margin of wing.
- (4)
- Tegmen apical setae longer than width of tegmen.
- (5)
- Tegmen apical plate curls transversely.
- (1)
- Transverse postocular groove.
- (2)
- Antennal insertion pointing ventrad.
- (3)
- One oval sensory pocket on each side of club antennomeres 1 and 2.
- (4)
- Procoxae not separated by septum.
- (5)
- Wing vein 1A1 present and shorter than 1A2.
- (6)
- Aedeagal internal sac with a dart.
- (7)
- Adults feeding primarily on pollen.
- (1)
- Single semicircular-shaped pit on each side of club antennomeres 1 and 2.
- (2)
- Pronotal maculation that extends to base of pronotum.
- (3)
- Tegmen with an apical visor that extends from the dorsal region to part of ventral margin.
- (4)
- Aedeagus dorsoventrally flattened.
- (5)
- Spermathecal tube covered with filaments (versus smooth in other Allocorynina [8]).
- (1)
- Wing vein 1A1 missing, but 1A2 and 3A retained.
- (2)
- Aedeagal apex subtruncate.
- (3)
- Aedeagal internal sac with transfer apparatus.
- (4)
- Tegmen with a dorso-lateral apical visor.
- (1)
- Male profemora with a single spine at base of profemoral apical pit.
- (2)
- Two elongate oval sensory pits on each side of club antennomeres 1 and 2.
- (3)
- Average scape length < length of funicular antennomeres 1 and 2.
- (1)
- Male profemora with pair of spines at either side of base of the profemoral apical pit.
- (2)
- Three round sensory pits on either side of club antennomeres 1 and 2.
- (1)
- Profemora without granules or spines.
- (2)
- Female RL/PL >1.27 and <1.44.
- (3)
- Length of aedeagal apex twice own width.
- (4)
- Larvae feed and pupate inside of male cone sporophylls.
- (1)
- Male profemora with granular field and spine.
- (2)
- Female RL/PL >1.77 and <1.95.
- (3)
- Larvae feed along cone axis.
- (4)
- Pupation outside of cone.
- (1)
- Male profemora with granular field and no spine.
- (2)
- Female RL/PL >1.55 and <1.76.
- (3)
- Larvae feed along cone axis.
- (4)
- Pupation outside of cone.
- (1)
- Profemora without granules or spines.
- (2)
- Female RL/PL >1.27 and <1.65.
- (3)
- Aedeagus with internal sac with ventral strut and dorsal pleats.
- (4)
- Larvae feed and pupate inside of male cone sporophylls.
Appendix D
References
- Norstog, K.; Stevenson, D.W.; Niklas, K.J. The role of beetles in the pollination of Zamia furfuracea L.fil. (Zamiaceae). Biotropica 1986, 18, 300–306. [Google Scholar] [CrossRef]
- Norstog, K.; Fawcett, P.K.S.; Vovides, A.P. Beetle pollination of two species of Zamia: Evolutionary and ecological considerations. Palaeobotanist 1992, 41, 149–158. [Google Scholar]
- Norstog, K.; Fawcett, P.K.S. Insect-cycad symbiosis and its relation to the pollination of Zamia furfuracea (Zamiaceae) by Rhopalotria mollis (Curculionidae). Am. J. Bot. 1989, 76, 1380–1394. [Google Scholar] [CrossRef]
- Tang, W. Insect pollination in the cycad Zamia pumila (Zamiaceae). Am. J. Bot. 1987, 74, 90–99. [Google Scholar] [CrossRef]
- Valencia-Montoya, W.A.; Tuberquia, D.; Guzmán, P.A.; Cardona-Duque, J. Pollination of the cycad Zamia incognita A. Lindstr. and Idárraga by Pharaxonotha beetles in the Magdalena Medio Valley, Colombia: A mutualism dependent on a specific pollinator and its significance for conservation. Arthropod-Plant Interact. 2017, 11, 717–729. [Google Scholar] [CrossRef]
- Vovides, A.P. Insect symbionts of some Mexican cycads in their natural habitat. Biotropica 1991, 23, 102–104. [Google Scholar] [CrossRef]
- Chaves, R.; Genaro, J. A new species of Pharaxonotha (Coleoptera: Erotylidae) probable pollinator of the endangered Cuban cycad, Microcycas calocoma (Zamiaceae). Insecta Mundi 2005, 19, 143–150. [Google Scholar]
- O’Brien, C.; Tang, W. Revision of the New World cycad weevils of the subtribe Allocorynina, with description of two new genera and three new subgenera (Coleoptera: Belidae: Oxycoryninae). Zootaxa 2015, 3970, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.S.; Nanni, I.; De Wet Bosenberg, J. The role of insects in the pollination of Encephalartos cycadifolius. In Proceedings of the Third International Conference on Cycad Biology; Vorster, P., Ed.; Cycad Society of South Africa: Stellenbosch, South Africa, 1995; pp. 423–434. ISBN 0620192283. [Google Scholar]
- Donaldson, J.S. Is there a floral parasite mutualism in cycad pollination? The pollination biology of Encephalartos villosus (Zamiaceae). Am. J. Bot. 1997, 84, 1398–1406. [Google Scholar] [CrossRef]
- Tang, W.; Oberprieler, R.; Yang, S. Beetles (Coleoptera) in cones of Asian Cycas: Diversity, evolutionary patterns, and implications for Cycas taxonomy. In Proceedings of the Fourth International Conference on Cycad Biology; Chen, C., Ed.; International Academic Publishers: Beijing, China, 1999; pp. 280–297. [Google Scholar]
- Tang, W. Cycad insects and pollination. In Vistas in Palaeobotany and Plant Morphology: Evolutionary and Environmental Perspectives Professor D.D. Pant Memorial Volume; Srivastava, P.C., Ed.; U.P. Offset: Lucknow, India, 2004; pp. 383–394. [Google Scholar]
- Terry, I. Thrips and weevils as dual, specialist pollinators of the Australian cycad Macrozamia communis (Zamaiceae). Int. J. Plant Sci. 2001, 162, 1293–1305. [Google Scholar] [CrossRef]
- Terry, I.; Tang, W.; Taylor, A.; Donaldson, J.; Singh, R.; Vovides, A.; Cibrián Jaramillo, A. An overview of cycad pollination studies. Mem. N. Y. Bot. Gard. 2012, 106, 352–394. [Google Scholar]
- Wilson, G.W. Pollination in the cycad genus Bowenia Hook. Ex Hook. f. (Stangeriaceae). Biotropica 2002, 34, 438–441. [Google Scholar] [CrossRef]
- Hall, J.A.; Walter, G.H.; Bergstrom, D.M.; Machin, P. Pollination ecology of the Australian cycad Lepidozamia peroffskyana (Zamiaceae). Aust. J. Bot. 2004, 52, 333–343. [Google Scholar] [CrossRef]
- Kono, M.; Tobe, H. Is Cycas revoluta (Cycadaceae) wind- or insect pollinated? Am. J. Bot. 2007, 94, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Procheş, Ş.; Johnson, S.D. Beetle pollination of the fruit-scented cones of the South African cycad, Stangeria eriopus. Am. J. Bot. 2009, 96, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Suinyuy, T.N.; Donaldson, J.S.; Johnson, S.D. Insect pollination in the African cycad, Encephalartos friderici-guilielmi Lehm. S. Afr. J. Bot. 2009, 75, 682–688. [Google Scholar] [CrossRef]
- Walters, T.; Osborne, R.; Decker, D. ‘We hold these truths’. In Cycad Classification Concepts and Recommendations; Walters, T., Osborne, R., Eds.; CABI Publishing: Oxfordshire, UK, 2004; pp. 1–11. ISBN 0851997414. [Google Scholar]
- Chemnick, J.; Oberprieler, R.; Donaldson, J.; Terry, I.; Osborne, R.; Tang, W.; Forster, P. Insect pollinators of cycads A report from a cycad pollination workshop held in Thailand, 2002 with a protocol for collecting and studying cycad pollinators. Cycad Newsl. 2004, 27, 3–7. [Google Scholar]
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Reid, C.A.M.; Schmitt, M.; Ślipińsk, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; O’Brien, C.W. Distribution and evolutionary patterns of the cycad weevil genus Rhopalotria (Coleoptera: Curculionoidea: Belidae) with emphasis on the fauna of Panama. Mem. N. Y. Bot. Gard. 2012, 106, 335–351. [Google Scholar]
- Calonje, M.; Stevenson, D.W.; Stanberg, L. The World List of Cycads. Available online: http://www.cycadlist.org (accessed on 2 May 2018).
- Franz, N.M.; Skelley, P. Pharaxonotha portophylla (Coleoptera: Erotylidae), new species and pollinator of Zamia (Zamiaceae) in Puerto Rico. Caribb. J. Sci. 2008, 44, 321–333. [Google Scholar] [CrossRef]
- Skelley, P.; Xu, G.; Tang, W.; Lindström, A.; Marler, T.; Khuraijam, J.S.; Singh, R.; Radha, R.; Rich, S. Review of Cycadophila Xu, Tang and Skelley (Coleoptera: Erotylidae: Pharaxonothinae) inhabiting Cycas (Cycadaceae) in Asia, with descriptions of a new subgenus and thirteen new species. Zootaxa 2017, 4267, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Skelley, P.; Thomas, M.C.; Perez-Farrera, M.A. Beetles inhabiting the cycad genus Ceratozamia (Cycadales: Zamaiceae). in preparation.
- Xu, G.; Tang, W.; Skelley, P.; Liu, N.; Rich, S. Cycadophila, a new genus (Coleoptera: Erotylidae: Pharaxonothinae) inhabiting Cycas debaoensis (Cycadaceae) in Asia. Zootaxa 2015, 3986, 251–278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maldonado-Ruiz, M.F.; Flores-Vázquez, J.C. Entomofauna Asociada a Dioon sp. nov. y actividad de los insectos polinizadores en San Jerónimo Taviche, Oaxaca, Mexico. Mem. N. Y. Bot. Gard. 2012, 106, 295–301. [Google Scholar]
- Oberprieler, R.G. The weevils (Coleoptera: Curculionoidea) associated with cycads. 2. Host specificity and implications for cycad taxonomy. In Proceedings of the 3rd International Conference of Cycad Biology, Conservation through Cultivation; Vorster; Vorster, P., Ed.; Cycad Society of South Africa: Stellenbosch, South Africa, 1995; pp. 335–365. ISBN 0620192283. [Google Scholar]
- Oberprieler, R.G. Evil weevils—The key to cycad survival and diversification? In Proceedings of the Sixth International Conference on Cycad Biology; Lindstrom, A., Ed.; Nong Nooch Tropical Garden: Bangkok, Thailand, 2004; pp. 170–194. ISBN 9749235916. [Google Scholar]
- Marvaldi, A.; Oberprieler, R.; Lyal, C.; Bradbury, T.; Anderson, R. Phylogeny of the Oxycoryninae sensu lato (Coleoptera: Belidae) and evolution of host-plant associations. Invertebr. Syst. 2006, 20, 447–476. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Farris, J.A.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Caldistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Wink, M.; Mikes, Z.; Rheinheimer, J. Phylogenetic relationships in weevils (Coleoptera: Curculionoidea) inferred from nucleotide sequences of mitochondrial 16S rDNA. Naturwissenschaften 1997, 84, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.B.; Cameron, S.A. Hierarchical analysis of variation in the mitochondrial 16S rRNA gene among Hymenoptera. Mol. Biol. Evol. 1998, 15, 1728–1743. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosoya, T.; Araya, K. Phylogeny of Japanese stag beetles (Coleoptera: Lucanidae) inferred from 16S mtrRNA gene sequences, with reference to the evolution of sexual dimorphism of mandibles. Zool. Sci. 2005, 22, 1305–1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sobti, R.S.; Sharma, V.L.; Kumari, M.; Gill, T.K. Genetic relatedness of six North-Indian butterfly species (Lepidoptera: Pieridae) based on 16S rRNA sequence analysis. Mol. Cell. Biochem. 2007, 295, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Aruggoda, A.G.B.; Shunxiang, R.; Baoli, Q. Molecular phylogeny of ladybird beetles (Coccinellidae: Coleoptera) inferred from mitochondrial 16S rDNA sequences. Trop. Agric. Res. 2010, 21, 209–217. [Google Scholar] [CrossRef]
- Caterino, M.S.; Cho, S.; Sperling, F.A.H. The current state of insect molecular systematics: A thriving tower of babel. Ann. Rev. Entomol. 2000, 45, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 9, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), version 4.0; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Nicolalde-Morejon, F.; Vergara-Silva, F.; Gonzalez-Astoraga, J.; Stevenson, D.W. Character based, population-level DNA barcoding in Mexican species of Zamia L. (Zamiaceae: Cycadales). Mitochondrial DNA 2010, 21, 51–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moynihan, J.; Vovides, A.P.; González-Astorga, J.; Francisco-Ortega, J. Population genetic diversity in the Dioon edule Lindl. species complex (Zamiaceae, Cycadales): Evidence from microsatellite data. Mem. N. Y. Bot. Gard. 2012, 106, 224–250. [Google Scholar]
- Moynihan, J.; Stevenson, D.W.; Lewis, C.E.; Vovides, A.P.; Caputo, P.; Francisco-Ortega, J. A phylogenetic study of Dioon Lindl. (Zamiaceae, Cycadales), based on morphology, nuclear ribosomal DNA, a low-copy nuclear gene, and plastid RFLPs. Mem. N. Y. Bot. Gard. 2012, 106, 448–479. [Google Scholar]
- Gutiérrez-Ortega, J.S.; Yamamoto, T.; Vovides, A.P.; Pérez-Farrera, M.A.; Martínez, J.F.; Molina-Freaner, F.; Watano, Y.; Kajita, T. Aridification as a driver of biodiversity: A case study for the cycad genus Dioon (Zamiaceae). Ann. Bot. 2018, 121, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Walter, G.H. Seed dispersal of the Australian cycad Macrozamia miquelii (Zamiaceae): Are cycads megafauna-dispersed “grove forming” plants? Am. J. Bot. 2013, 100, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Burbridge, A.H.; Whelan, R.J. Seed dispersal in acycad, Macrozamia riedlei. Aust. J. Ecol. 1982, 7, 63–67. [Google Scholar] [CrossRef]
- Ballardie, R.T.; Whelan, R.J. Masting, seed dispersal and seed predation in the cycad Macrozamia communis. Oecologia 1986, 70, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tang, W. Seed dispersal in the cycad Zamia pumila in Florida. Can. J. Bot. 1989, 67, 2066–2070. [Google Scholar] [CrossRef]
- Crowson, R.A. On the systematic position of Allocoryninae (Coleoptera: Allocorynidae). Coleopt. Bull. 1986, 40, 243–244. [Google Scholar]
- Crowson, R.A. The relations of Coleoptera to Cycadales. In Advances in Coleopterology; Zunino, M., Belle’s, X., Blas, M., Eds.; Asociacion Europea de Coleopterologia: Torino, Italy, 1991; pp. 13–28. [Google Scholar]
- Brookes, D.R.; Hereward, J.P.; Terry, L.I.; Walter, G.H. Evolutionary dynamics of a cycad obligate pollination mutualism—Pattern and process in extant Macrozamia cycads and their specialist thrips pollinators. Mol. Phylogenet. Evol. 2015, 93, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Franz, N.M.; Engel, M.S. Can higher-level phylogenies of weevils explain their evolutionary success? A critical review. Syst. Entomol. 2010, 35, 596–606. [Google Scholar] [CrossRef]
- Rogers, R.D.; Mann, P.; Emmet, P.A. Tectonic terranes of the Chortis block based on integration of regional aeromagnetic and geologic data. In Geologic and Tectonic Development of the Caribbean Plate in Northern Central America; Mann, P., Ed.; Geological Society of America: Boulder, CO, USA, 2007; Volume 428, pp. 65–88. [Google Scholar] [CrossRef]
- Coates, A. The forging of Central America. In Central America: A Natural and Cultural History; Coates, A., Ed.; Yale Univ. Press: New Haven, CT, USA, 1997; pp. 1–37. ISBN 0300068298. [Google Scholar]
- Tang, W. Evolutionary history of cycads in North America. Cycad Newsl. 2012, 35, 7–13. [Google Scholar]
- Kvaček, Z. A noteworthy cycad, Ceratozamia hofmannii Ettingshausen 1887, from the Lower Miocene of Austria re-examined. Neues Jahrb. Geol. Paläontol. Monatshefte 2004, 111–118. [Google Scholar]
- Nagalingum, N.; Marshall, C.; Quental, T.; Rai, H.; Little, D.; Matthews, S. Recent synchronous radiation of a living fossil. Science 2011, 334, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Salas-Leiva, D.; Meerow, A.W.; Calonje, M.; Griffith, M.P.; Francisco-Ortega, J.; Stevenson, D.W.; Nakamura, K.; Lewis, C.E.; Namoff, S. Phylogeny of the cycads based on multiple single copy nuclear genes: Congruence of concatenation and species tree inference methods. Ann. Bot. 2013, 112, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Condamine, F.; Nagalingum, N.; Marshall, C.R.; Morlon, H. Origin and diversification of living cycads: A cautionary tale on the impact of the branching process prior in Bayesian molecular dating. BMC Evol. Biol. 2015, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Meerow, A.W.; Francisco-Ortega, J.; Calonje, M.; Griffith, M.P.; Ayala-Silva, T.; Stevenson, D.W.; Nakamura, K. Zamia (Cycadales: Zamiaceae) on Puerto Rico: Asymmetric genetic differentiation and the hypothesis of multiple introductions. Am. J. Bot. 2012, 99, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Lara, J.C. The Reproductive Biology of Zamia (Cycadales: Zamaiaceae) in Puerto Rico: Implications for Patterns of Genetic Structure and Species Conservation. Ph.D. Thesis, University of Puerto Rico, Rio Píedras, Puerto Rico, May 2015. [Google Scholar]
- Caputo, P.; Cozzolino, S.; De Luca, P.; Moretti, A.; Stevenson, D.W. Molecular Phylogeny of Zamia (Zamiaceae). In Cycad Classification Concepts and Recommendations; Walters, T., Osborne, R., Eds.; CABI Publishing: Oxfordshire, UK, 2004; pp. 149–157. ISBN 085199741. [Google Scholar]
- Nagalingum, N.; Marshall, C.; Quental, T.; Rai, H.; Little, D.; Matthews, S. Supporting Online Material for Recent Synchronous Radiation of a Living Fossil. Science 2011, 1–38. [Google Scholar] [CrossRef]
- Salas-Leiva, D.E.; Meerow, A.W.; Calonje, M.; Francisco-Ortega, J.; Griffith, M.P.; Nakamura, K.; Sánchez, V.; Knowles, L.; Knowles, D. Shifting Quaternary migration patterns in the Bahamian archipelago: Evidence from the Zamia pumila complex at the northern limits of the Caribbean island biodiversity hotspot. Am. J. Bot. 2017, 104, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Norstog, K.; Nicholls, T. The Biology of the Cycads; Cornell Univ. Press: Ithaca, NY, USA; London, UK, 1997; 383p, ISBN 080143033X. [Google Scholar]
- Donaldson, J. Cycads Status Survey and Conservation Action Plan; IUCN: Gland, Switzerland; Cambridge, UK, 2003; 86p, ISBN 2831706998. [Google Scholar]
- Poinar, G.; Legalov, A.A. Pleurambus strongylus n. gen., n. sp. (Coleoptera: Belidae) in Dominican amber. Hist. Biol. 2014, 26, 670–674. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Bukejs, A. First fossil representatives of Pharaxonothinae Crowson (Coleoptera: Erotylidae): Indirect evidence for cycads existence in Baltic amber forest. Zootaxa 2017, 4337, 413–422. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Xu, G.; O’Brien, C.W.; Calonje, M.; Franz, N.M.; Johnston, M.A.; Taylor, A.; Vovides, A.P.; Pérez-Farrera, M.A.; Salas-Morales, S.H.; et al. Molecular and Morphological Phylogenetic Analyses of New World Cycad Beetles: What They Reveal about Cycad Evolution in the New World. Diversity 2018, 10, 38. https://doi.org/10.3390/d10020038
Tang W, Xu G, O’Brien CW, Calonje M, Franz NM, Johnston MA, Taylor A, Vovides AP, Pérez-Farrera MA, Salas-Morales SH, et al. Molecular and Morphological Phylogenetic Analyses of New World Cycad Beetles: What They Reveal about Cycad Evolution in the New World. Diversity. 2018; 10(2):38. https://doi.org/10.3390/d10020038
Chicago/Turabian StyleTang, William, Guang Xu, Charles W. O’Brien, Michael Calonje, Nico M. Franz, M. Andrew Johnston, Alberto Taylor, Andrew P. Vovides, Miguel Angel Pérez-Farrera, Silvia H. Salas-Morales, and et al. 2018. "Molecular and Morphological Phylogenetic Analyses of New World Cycad Beetles: What They Reveal about Cycad Evolution in the New World" Diversity 10, no. 2: 38. https://doi.org/10.3390/d10020038
APA StyleTang, W., Xu, G., O’Brien, C. W., Calonje, M., Franz, N. M., Johnston, M. A., Taylor, A., Vovides, A. P., Pérez-Farrera, M. A., Salas-Morales, S. H., Lazcano-Lara, J. C., Skelley, P., Lopez-Gallego, C., Lindström, A., & Rich, S. (2018). Molecular and Morphological Phylogenetic Analyses of New World Cycad Beetles: What They Reveal about Cycad Evolution in the New World. Diversity, 10(2), 38. https://doi.org/10.3390/d10020038




