Abstract
Translocations are stressful, especially when captive animals are naïve to natural stimuli. Captive eastern hellbenders (Cryptobranchus alleganiensis alleganiensis) identify predatory fish as threats, but may be more vulnerable to predation and stress because of inexperience with them. We investigated the use of predator conditioning to prepare hellbenders, behaviorally and physiologically, for the presence of a common predator, largemouth bass (Micropterus salmoides). We reared hellbenders for 30 d with and without continuous exposure to largemouth bass kairomones and heterospecific alarm cues and found conditioned hellbenders became less active compared to unconditioned individuals (p = 0.017). After conditioning, we exposed hellbenders to water, a low concentration of kairomones, or a high concentration of kairomones in a closed respirometer system. We measured activity within respirometer chambers and routine metabolic rate. We found unconditioned hellbenders exposed to low and high concentrations of kairomones were 41% and 119% more active than conditioned animals (p = 0.002 and p < 0.001). Moreover, conditioned individuals had on average 6.5% lower metabolic rates across all three kairomone concentrations compared to unconditioned individuals (p = 0.017). Our data suggest that predator conditioning induces behavioral avoidance tactics and physiological changes that could improve future translocation efforts for hellbenders and other imperiled species.
1. Introduction
Translocations are inherently stressful for animals [1,2,3]. Not only is transportation and release into a novel environment challenging, but stress is further exacerbated by exposure to additional threats that are typically absent from the captive environment. Stimuli such as stochastic weather conditions, contaminants, pathogens, and predators are novel to captive-reared animals and can magnify the stress of translocations [1,4,5,6]. Increased stress is correlated with reduced reproductive potential, increased disease susceptibility, altered energy expenditure, irregular dispersal movements, and increased predation risk [7,8]. Subsequently, stress (in a variety of forms) is a leading cause of translocation mortality [1,2,3,9,10].
Animals reared in captivity are often naïve to predators and can lack experience in predator detection and appropriate avoidance responses [11]. Subsequently, captive-reared animals have higher mortality rates than wild or predator-conditioned conspecifics [12]. For example, captive-reared partridges (Perdix perdix) have a 69% chance of predator-mediated mortality following reintroduction because of inappropriate, or absent, antipredator behaviors [13]. Predators can lethally remove individuals from the population, but can also cause considerable physiological and psychological stress in their prey [14]. Tadpoles (Rana clamitans and R. catesbeiana) reared with caged dragonfly (Anax junius) predators have increased mortality rates because the presence of a predator can cause physiological stress that leads to death even without a physical encounter [15]. Predator detection is associated with an increase in heart rate, elevated respiration, and the release of stress hormones (i.e., glucocorticoids), which are all components of the fight or flight response [16,17,18,19]. Although acute fright responses are adaptive in responding to predators, chronic activation of the fright response becomes maladaptive during extended exposure or in combination with other stressful stimuli (e.g., translocation [20]).
Repeated predator-exposure events can familiarize an individual to reoccurring threats and prepare them physiologically to not respond with continuous activation of their acute stress response [21]. Furthermore, repeated exposures can train animals to appropriately assess their level of risk and better balance predator avoidance with energy allocation [22,23]. Fright responses are energetically costly; therefore, some fish exposed to predators conserve energy during low stress events to reserve resources for more threatening scenarios [24]. Moreover, animals conditioned to chronic stress or living in areas with high densities of predators have more transient responses to threatening stimuli, exhibit lower levels of circulating stress hormones, and recover from acute stress events quicker [24,25,26]. Although presenting predators to naïve prey can be initially stressful, prey species experience repeated predator exposure events in the wild and must alter their physiological demands and avoidance strategies to successfully coexist with predators. If predator conditioning prior to release can reduce predator-mediated stress and prepare captive animals to identify and avoid novel predators, perhaps animals will be better able to manage the energetic costs associated with the stress of transportation and wild release [3].
Eastern hellbender (Cryptobranchus alleganiensis alleganiensis) translocations, from captivity to the wild, have resulted in variable levels of success (17–72% survival over six months; [27,28,29]). Translocation failures have been attributed to disease, long distance dispersal, and predation, which are all inherently linked with stress. Hellbenders are fully aquatic and reside in rivers with a diverse array of predatory fish species (see [30]) and thus, an abundance of predator kairomones—chemical cues emitted by predatory species. Largemouth bass (Micropterus salmoides) live in sympatry with hellbenders in some rivers and are capable of consuming large prey items, such as 1–3 year old hellbenders [31]. Young, captive hellbenders respond to predatory fish kairomones with altered behavior, suggesting that they accurately identify kairomones as stressful stimuli and have innate recognition to predators, such as largemouth bass [11,32]. Translocations into predator-rich environments could exacerbate stress from transport and release and become detrimental to hellbenders; however, there has been no research to identify whether hellbenders have physiological responses to predators. In order to increase translocation success, it is important that hellbenders are able to identify, assess, and respond to predatory risk with advantageous avoidance behaviors, but without activation of physiologically costly stress responses and metabolic demands. We investigated the ability of juvenile hellbenders to detect predators, and their foraging and behavioral responses over acute periods of exposure, and their physiological response to low and high levels of risk after being conditioned to largemouth bass kairomones. We predicted that conditioning captive, juvenile hellbenders with predator kairomones would improve their ability to detect predators, increase their use of refugia and behavioral avoidance strategies, and reduce physiological stress.
2. Materials and Methods
2.1. Study Animals
Eastern hellbender salamanders are threatened or endangered throughout much of their range in the central and southeastern United States. They are state-endangered in Indiana, USA and restricted to a single river system [33]. In efforts to preserve and bolster this wild population, researchers have been collecting hellbender clutches from the wild, head-starting them in captivity, and releasing them back into their natal river at older and larger age classes. We collected one wild clutch of eggs, all at least half siblings, in 2015. These individuals (n = 122) were reared in multiple aquarium tanks with sterilized water, tile hides, limited stimuli, and standardized food regimes for the first two years of their life. We haphazardly selected 48 of these individuals for this project, as all of them were completely naïve to predator kairomones. All animal handling procedures were reviewed and approved by Purdue University’s Animal Care and Use Committee (protocol number 1406001094, approved 05/2017).
2.2. Phase 1. Predator Conditioning
We reared two-year-old eastern hellbenders for 30 d after randomly assigning them to one of two conditioning treatments, with (conditioned, n = 24) or without (unconditioned, n = 24) continuous exposure to largemouth bass (Micropterus salmoides) kairomones (similar to [19]). We housed three largemouth bass in separate tanks directly above conditioned treatment tanks. We created a gravitational flow-through design, such that water from tanks with predators entered into the hellbender treatment tanks directly below them (similar to [34]). All tanks continually received fresh filtered and ultraviolet-sterilized well water (20 ± 2 °C); however, because conditioning tanks also received predator tank water, they were continually exposed to low concentrations of predator kairomones. We fed the bass live larval tiger salamanders (Ambystoma tigrinum) each day in order to provide salamander alarm cues—chemical cues emitted by prey during stress, disturbance, or attack—in conjunction with predator kairomones. Predator conditioning that combines predator kairomones with damage-released alarm cues, or some kind of aversive stimuli, can facilitate predator recognition and reduces the likelihood of naïve prey becoming habituated to kairomones [35]. It was important to provide an amphibian warning signal because amphibians elicit stronger responses when predators are fed amphibian prey [23,36,37]. However, we were unable to sacrifice hellbenders because of their endangered conservation status and we required more than 180 prey individuals. Subsequently, we used larval tiger salamanders because we could easily acquire multiple egg clutches prior to the experiment and this provided us a source of amphibian alarm cues.
We weighed all hellbenders at the beginning and end of the conditioning period; all hellbenders were comparable in size between the two treatments (mean = 42.2 g, Standard Deviation ± 8.8 g, t = −0.69, p = 0.491). We housed eight hellbenders per tank with a total of six tanks. We conducted behavioral observations 21 times over the 30 conditioning days, or 5–6 times a week during daytime hours (0800–1700 h). We conducted scan sampling to count the number of individuals outside tile hides [38]. Of those individuals outside the refugia, we classified and counted the number of individuals actively moving, stationary, or floating in the tank. We selected these three groupings, as they categorized behaviors commonly observed outside of the tile hides in captivity. We provided each hellbender tank with 20 g of black worms (Lumbriculus variegatus), twice weekly. At each feeding event we recorded the time taken for at least one hellbender to start eating and noted the total number of individuals emerging from hides to feed within 10 min of providing food. At the beginning of each week, prior to feedings, we removed and weighed any worms that were remaining in the tanks to estimate overall consumption.
2.3. Phase 2. Exposure Trials
Following 30 d of conditioning, we randomly assigned hellbenders to three exposure treatments for a full 2 × 3 factorial design: conditioned or unconditioned treatments crossed with control (no kairomones), low risk (low concentration of largemouth bass kairomones), or high risk (high concentration of largemouth bass kairomones) exposures. This design allowed us to compare the physiological responses of hellbenders chronically exposed to low risk and then (1) released from predator threat; (2) maintained in a chronic low risk environment; or (3) exposed to a novel high risk environment. We were also able to compare the response of unconditioned, naïve individuals exposed to these three levels of risk.
Predators are physiologically demanding to prey species. Their physical or chemical presence increases circulating glucocorticoid levels, induces altered behaviors, and changes respiration rates [34,39,40]. Metabolic rate is directly tied to the stress response; therefore, it provides a reliable metric for a physiological response [17]. We used largemouth bass as our focal threat and then exposed all hellbenders to predator kairomones within respirometer chambers to measure changes in the routine metabolic rate. We measured aquatic oxygen consumption using a Loligo Systems closed respirometer (Viborg, Denmark). The system consisted of four cylindrical glass chambers, each with a Witrox 4 for oxygen and temperature readings. The four chambers were connected to two pumps each via impermeable plastic tubing. The first pump moved fresh water into the chambers while the second pump recirculated water past the oxygen sensor that recorded readings every 30 s. All oxygen sensors were calibrated to 0% O2 using sodium-sulfite-treated water and 100% O2 using fully aerated water [41]. We submerged all chambers, tubing, and pumps in a large 180-gallon sump full of UV-sterilized water [42]. In order to add predator kairomones for the low and high risk exposure treatments, we added predatory fish directly to the respirometer holding sump: one fish in 75 gallons of water for low risk and three fish in 75 gallons of water for high risk. Adding additional predators increases the concentration of kairomones in the water and, therefore, could increase perceived levels of risk [43]. We allowed the fish to swim around the holding tank for one hour, removed the fish, and then started the hellbender respirometer trials. We did not provide any alarm cues during the exposure trials, only predator kairomones.
Prior to exposure trials, we fasted all hellbenders for 48 h to reach a post-absorptive state [42,44,45]. We also acclimated hellbenders in open circuit respirometers for five minutes then created a closed, recirculating circuit for each individual chamber [41]. Hellbenders are primarily nocturnal; therefore, we conducted all experiments during daylight hours and kept overhead lights on in the experimental room to reduce activity. We conducted experiments over two days, between 1000 and 1600 h, wherein we tested all individuals exposed to well water and then all individuals exposed to largemouth bass kairomones to avoid contamination between groups. We started with low risk exposure and then tested high risk exposure within the same day. Again, we elected not to randomize our testing in order to avoid contamination across the three risk levels. We ran trials with a single hellbender in each of the four chambers for 30 min. We restricted the sampling time to 30 min to avoid creating hypoxic conditions (<3 mgO2 L–1) within the closed system while still allowing enough time to detect reductions in oxygen concentrations [46]. We maintained equal temperatures across trials, which were comparable to the hellbenders’ rearing environment and to previous studies [47]. We used the software package AutoResp to detect the oxygen consumption of each chamber in real time (mL O2 h–1, Loligo Systems, Viborg, Denmark).
Subtle changes in behavior can cause increases in metabolic rate; therefore, we wanted to account for any activity within the chamber during our exposure trials. There were no hides added to the respirometer chambers because of limited space, which allowed us to fully observe each hellbender throughout the duration of the exposure trials. We scan sampled all four respirometer chambers every minute and recorded whether hellbenders were moving as a binary response. Moving could include turning around, rocking body, or swaying tail. We then combined the total number of times a hellbender was observed active during the 30 min trial to calculate the proportion of time active.
2.4. Statistical Analyses
2.4.1. Phase 1: Predator Conditioning
We made statistical comparisons of refuge use and tank behavior between conditioned and unconditioned individuals over the 30 d conditioning period. We tested for differences in the number of individuals outside the tile refugia during each behavioral observation using a generalized linear mixed-effects model. We used Poisson distribution, because we found no evidence of overdispersion after using the ‘AER’ package in R. We included ‘treatment’ and ‘date’ in our model, tested for an interaction between the two, ‘treatment*date’, and accounted for repeated observations by also including ‘rearing tank’ nested within ‘date’ as random effects. We used a multivariate analysis of variance (MANOVA) to compare the tank behavior of hellbenders observed outside of the refuge between conditioned and unconditioned treatments. We centered and scaled the number of hellbenders active, stationary, or floating in the tank to meet the assumption of normality and then combined them as multivariate response variables [48]. We tested for behavioral differences across time of day, but found time to be unimportant and removed it from all models (f value = 0.55, p = 0.647). We then conducted repeated measure ANOVAs to compare each tank’s behavior (i.e., active, stationary, and floating) between conditioning treatments. We included ‘rearing tank’ nested within ‘date’ to account for repeated measures through time and tested for ‘treatment’, ‘date’, and ‘treatment*date’ interaction effects.
We compared feeding behavior with two separate analyses, using generalized linear mixed-effects models that accounted for repeated observations through time (i.e., ‘rearing tank’ nested in ‘date’). We compared the time to start feeding between conditioned and unconditioned individuals using a Gaussian distribution, and the total number of individuals observed feeding using a Poisson distribution. In addition, we used linear mixed-effects models to test for differences in the amount of food eaten each week; again, we treated ‘rearing tank’ nested in ‘date’ as a random effect. We also conducted t-tests to compare the mass of conditioned and unconditioned individuals at the end of the conditioning period.
2.4.2. Phase 2: Exposure Trials
We tested for differences in activity between conditioned and unconditioned hellbenders during predator exposure trials in respirometer chambers. We included the proportion of time active as our response variable and conducted binomial logistic regressions using the total number of times hellbenders were observed active or stationary during the exposure trials. We compared the effects of conditioning, exposure level, and their interactions on the probability of moving.
We were interested in how our measure of activity related to metabolic rate; therefore, we conducted a linear regression with metabolic rate as a function of proportion of time active. We found the proportion of time active to be highly significant (t value = 5.80, p < 0.001, Figure 1) and chose to include it in all metabolic rate comparisons as a way to account for activity in our metabolic rate models. We compared metabolic rate between conditioned and unconditioned individuals at each exposure treatment using analysis of covariance (ANCOVA) with ‘mass’ and ‘proportion of time active’ as covariates. We considered one individual hellbender to be an outlier, because it was double the mean weight of all other hellbenders, and removed it from metabolic rate comparisons. All variables met assumptions of normality except mass, which we log-transformed. We tested for a mass by treatment effect in the model, but did not find evidence for an interaction between treatment and mass (t value = −1.12, p = 0.271). We also tested for interactions between conditioning treatment and exposure level, after correcting for mass and accounting for proportion of time active. We tested for any effects of time on the probability of moving within chambers or on metabolic rate, but excluded this variable from our final models, as it was not a significant predictor for either response (probability of moving: t value = −1.08, p = 0.286; metabolic rate: t value = −0.22, p = 0.824). We used the program R, version 3.2.3, for all analyses with an alpha level of 0.05 [49]. We used package ‘emmeans’ to report marginal means and standard errors around metabolic rate estimates. All data files are stored on Purdue University’s Research Repository (http://purr.purdue.edu).
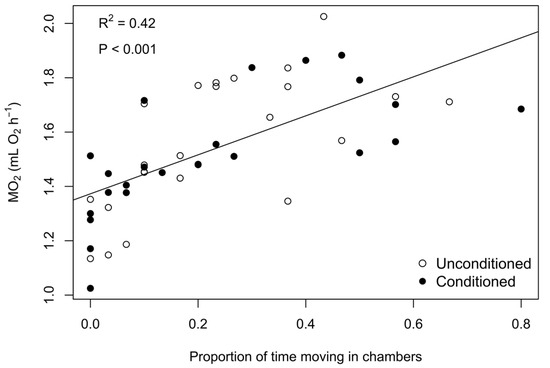
Figure 1.
Linear relationship between routine metabolic rate and proportion of time moving in respirometer chambers. The added regression line shows the strong relationship between general activity (i.e., turning, rocking, or moving) and oxygen consumption during exposure trials.
3. Results
3.1. Phase 1: Predator Conditioning
We found no differences in the number of hellbenders outside of refugia between conditioning treatments (estimated difference = −0.08, z value = −0.60, p = 0.55); however, we did find significant differences across sampling days (estimated change per day = −0.07, z value = −5.12, p < 0.001). All hellbenders, regardless of their treatment, increased their refuge use during the length of the experimental period. Of the individuals that we observed outside of refugia, we detected significant multivariate differences in active, stationary, or floating behaviors between conditioning treatments (p < 0.001) as well as a treatment*date interaction (p = 0.012; Table 1). Furthermore, we detected behavioral differences between treatments depended on sampling date, such that conditioned individuals were less likely to be active or float in tanks, compared to unconditioned individuals, as the conditioning period progressed (Table 1, Figure 2).
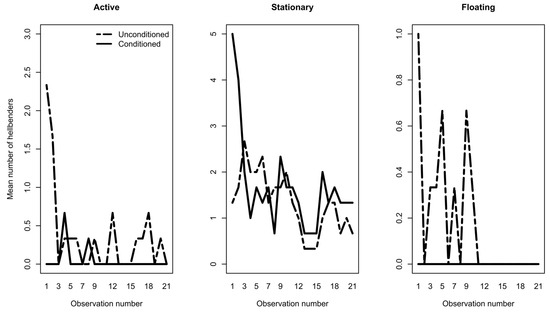
Figure 2.
Behavioral observations of unconditioned and conditioned hellbenders in rearing tanks during the 30 d conditioning period. Hellbenders were counted whenever observed outside of tile hides and categorized as active, stationary, or floating. The mean number of hellbenders observed active or floating through time depended on conditioning treatment, such that fewer conditioned individuals were observed active or floating through the duration of the conditioning period compared to unconditioned individuals (p < 0.05). There were no significant treatment or time effects on stationary behavior.
We found no differences in the time to start feeding (difference = 0.69 min, t value = 1.00, p = 0.335) or the number of individuals observed feeding within a 10 min observation period (estimated difference = 0.83 individuals, z value = −0.54, p = 0.587). Furthermore, there was no difference in the amount of worms eaten between treatments (estimated difference = 2.18 g, t value = 0.57, p = 0.581). However, hellbenders conditioned to largemouth bass kairomones weighed 11.9% more (95% Confidence Interval = 0.03–24%) at the end of the conditioning period compared to unconditioned hellbenders (estimated difference = 5.53 g, t value = −2.02, p = 0.049).
3.2. Phase 2: Exposure Trials
There were significant differences in the probability of moving during the predator exposure respirometer trials between conditioned and unconditioned individuals. Conditioned individuals were 15.4% more likely to move in respirometer chambers when exposed to water without predator kairomones (estimated difference in probability of moving = 0.06, z value = 2.90, p = 0.004; Figure 3). However, they were 28.9% and 54.3% less likely to move compared to unconditioned individuals in low and high exposure trials, respectively (estimated difference in probability of moving = 0.05, z value = −3.03, p = 0.002 and estimated difference in probability of moving = 0.10, z value = −4.29, p < 0.001; Figure 3).
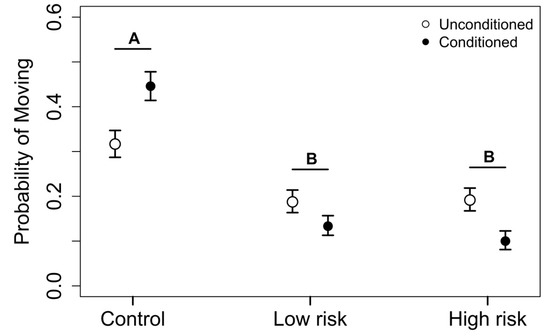
Figure 3.
The probability of moving within respirometer chambers during exposure to varying levels of risk/kairomone concentrations. Activity was measured as proportion of time turning, rocking the body, or moving the tail during the 30 min exposure trial. Conditioned hellbenders increased their probability of moving when exposed to water without kairomones, but had a lower probability of moving when they were presented with low and high concentrations of predator kairomones compared to unconditioned individuals (p < 0.05). Estimates are back-transformed and presented with standard errors. There were significant differences between conditioned and unconditioned individuals at each of the three exposure levels. Letters indicate significant differences across exposure levels (p < 0.05).
Conditioned individuals had significantly lower metabolic rates compared to unconditioned individuals across all three exposure levels, even after accounting for the proportion of time active in respirometer chambers (t value = −2.49, p = 0.017; Figure 4). Conditioned hellbenders exposed to water without kairomones had 6.7% lower metabolic rates compared to unconditioned, control individuals (estimated difference in mL O2 h–1= 0.10, t value = −2.49, p = 0.017; Figure 4). Furthermore, conditioned individuals exposed to low and high risk had metabolic rates 6.4% lower than unconditioned individuals (estimated difference in mL O2 h–1 = 0.10, t value = −2.49, p = 0.017; Figure 4).
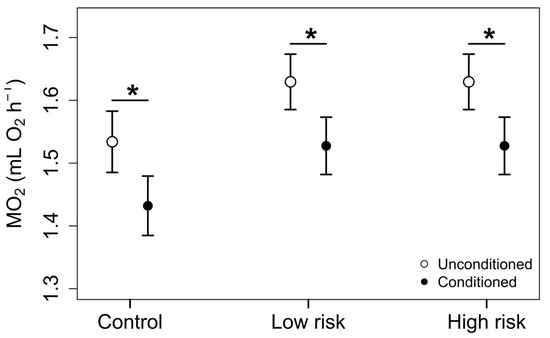
Figure 4.
Oxygen consumption (mL O2h–1) of conditioned and unconditioned hellbenders exposed to varying levels of kairomone concentrations, after accounting for mass and proportion of time active during the exposure trials. Estimates are presented as marginal means with standard errors. Conditioned individuals had consistently lower oxygen consumption at each of the exposure levels compared to unconditioned individuals, but the rate of oxygen consumption did not differ across exposure levels. Asterisks denote significant differences between conditioning treatments (p < 0.05).
4. Discussion
Predator conditioning induced predator avoidance behaviors and provided strong evidence that hellbenders perceive largemouth bass kairomones as a threat. Hellbenders conditioned to largemouth bass kairomones and heterospecific alarm cues for 30 d were less active outside of tile hides and never observed floating compared to control hellbenders that only received well water. All hellbenders increased their refuge use through the duration of the conditioning period, which suggests that hellbenders became acclimated to the rearing tanks and reduced their exploratory behavior. However, conditioned individuals demonstrated behavioral plasticity with chronic exposure to predator kairomones by reducing their time moving outside of refuge over time. Some of the most common predator avoidance strategies, observed across a multitude of taxonomic groups, are decreased movement, freezing in place, or seeking out refuge. For example, less mobile voles (Microtus agrestis) have reduced rates of predator capture, small-mouth salamanders (Ambystoma texanum) spend more time in refuge away from green sunfish (Lepomis cyanellus), and crayfish (Orconectes rusticus) freeze in place to be less conspicuous [34,50,51]. Behavioral responses reduce the probability of being detected, encountering predators, or being captured, and are adaptive when coexisting with predators. Remaining stationary is especially beneficial for hellbenders in the presence of fish predators, because fish use a lateral line system to detect and locate prey through movement [52]. Alternatively, floating in the water column or at the water surface likely increases the risk of capture by an aquatic or terrestrial predator and potentially being swept downstream in riverine water currents. This maladaptive behavior is commonly observed among hellbenders in captivity and could be particularly threatening to hellbenders’ survival in the wild. However, conditioned individuals were never observed floating in their tanks, suggesting that predator conditioning induced plastic behaviors that will aid in predator avoidance rather than predator capture.
Animals can suffer reduced growth in the presence of predation risk if they face a trade-off between foraging to fulfill their energy needs and remaining inactive to avoid predation (i.e., the growth/predation tradeoff [53]). Although conditioned hellbenders were less active in their tanks, we did not observe differences in foraging behavior or overall food intake. Moreover, conditioned individuals were able to gain more weight during the conditioning period. Inactivity is inherently linked to reduced energy expenditure, and can also be associated with higher somatic growth [54]. For example, fish reared with predators have 80% greater mean weight than controls, because they expend less energy during periods of inactivity and are able to allocate resources beyond general maintenance costs [54]. Conditioned hellbenders may have been more stealthy feeders, reducing extraneous exploratory behavior when foraging and instead directing resources to growth. Additionally, some animals allocate resources to develop morphological defenses in the presence of predators, such as spines, crush-resistant shells, or body sizes that are beyond the gape limitation of predators [55,56,57]). Hellbender weight gain provides evidence that predator conditioning was not detrimental to growth and refutes arguments for a tradeoff between growth and behavioral avoidance of predation in this experiment. Furthermore, larger hellbenders may be more likely to survive in the wild and to survive longer following release, as size is often a positive predictor of post-release success (see [58]). Hellbenders are a slow-growing species, yet within 30 d the conditioned hellbenders gained weight. This suggests that conditioned hellbenders were better able to direct resources toward growth and that this technique can effectively increase size prior to release.
Following the conditioning period, we found that both conditioned and unconditioned individuals reduced their level of activity when they were exposed to largemouth bass kairomones in respirometer chambers. These results substantiate other work that found that amphibians, including hellbenders, have innate behavioral responses to predator kairomones [32,59,60]. Crane and Mathis [11] found that larval hellbenders, 21–25 weeks old, increase their swimming when exposed to trout kairomones, which they interpreted as evidence of escape behavior. Our findings differ from Crane and Mathis [11], likely because we presented live amphibian prey to largemouth bass as food. Although their study elicited responses using hellbender slime as an alarm cue, they fed their fish a diet of floating trout feed. Disturbance cues released from injured or stressed prey induce predator defenses and phenotypic plasticity among conspecifics [43,61,62,63]. However, cues from chewed and digested prey have an even stronger influence on predator avoidance strategies; tadpoles exposed to predators fed conspecifics reduce their activity by 30% compared to tadpoles only exposed to a starved predator [64]. We observed different predator avoidance behaviors, likely because of the diet of largemouth bass. Furthermore, in the wild, larvae are reared in a nest that is paternally guarded and remain for months after hatching (5–6 m, W. Hopkins Personal communication). Therefore, larvae between 21 and 25 weeks old may have underdeveloped predator avoidance strategies because of innate protection from potential predators in the nest. Alternatively, our design more closely mimicked a natural environment where two-year-old hellbenders would be free swimming in the river, continually exposed to predator kairomones, and needing to actively avoid predation. Conditioned hellbenders in our study responded with stronger reductions in activity than unconditioned individuals, suggesting that predator conditioning for captive hellbenders might reduce susceptibility to predation and possible sublethal effects following translocation to the wild. Conditioned and unconditioned hellbenders reduced their activity by 70.1% and 40.8%, respectively, when presented with kairomones from one largemouth bass, but we did not observe further changes in chamber activity after exposure to high concentrations of kairomones. A threshold response following the addition of one predator is similar among other amphibians [65,66]. Wood frog tadpoles (Rana sylvatica) reduce their activity by 38% when presented with a single predator, but then show no additional differences in activity when two, four, or six predators are presented [65]. Oppositely, pool frog tadpoles increase the proportion of inactive individuals by 22% when they are presented with a single caged predator, but have no additional changes when three more caged predators are added to the same holding tank [66]. Regardless of the magnitude of risk, conditioned hellbenders had consistently lower chamber activity in the low and high concentration trials, which translates to higher energy savings compared to unconditioned individuals. This could quickly become useful if hellbenders need to flee or escape lethal predators, leading to a survival advantage over predator-naïve hellbenders released into the wild [19,24].
Being able to assess and opportunistically respond to the presence or absence of risk supports the risk allocation hypothesis [67]. This hypothesis suggests that animals decrease their levels of activity when they detect high risk, but increase their foraging or activity during bouts of perceived safety [67,68]. For example, snails (Physa gyrina) that are maintained at high levels of risk and then exposed to a pulse of safety increase their activity until the pulse of low risk passes [68]. Conditioned hellbenders exposed to water during the exposure trials moved more than unconditioned hellbenders in the respirometer chambers. This release from predator pressure may have been perceived as a bout of safety and induced more activity. We did not detect differences in activity between conditioned individuals in low and high exposure treatments. However, this might be attributed to the fact that we did not present salamander alarm cues in combination with the fish kairomones during the exposure trials. Hellbenders can recognize conspecific alarm cues and perceive it as an indicator of elevated risk [11]. Therefore, adding hellbender slime or alarm cues from other salamanders could have exaggerated behavioral responses. Despite this, conditioned individuals had lower activity in the low and high exposure treatments compared to unconditioned individuals and demonstrate a conditioning benefit even in the absence of conspecific or heterospecific alarm cues.
We are the first to observe physiological responses to largemouth bass kairomones in eastern hellbenders and confirm that predator conditioning successfully minimizes hellbenders’ energetic demands. Conditioned hellbenders had on average 6.5% lower metabolic rates compared to unconditioned individuals, even after accounting for the effects of activity within the respirometer chambers. Similarly, common frog (Rana temporaria) tadpoles have 10% lower oxygen consumption rates after being exposed to predator kairomones for 30 d [19]. In addition, Arabian toad (Bufo arabicus) tadpoles reared with continuous exposure to dragonfly (Anax sp.) larvae show a linear decrease in their respiration and had metabolic rates ~45% lower than controls after 21 d of conditioning [69]. Lower metabolic baselines correlate with lower energetic demand and better budgeting of available resources under chronic risk [19,21]. Maintaining a lower metabolic rate is highly advantageous in a risky environment, because animals are able to avoid excessive energy expenditure, minimize anti-predator costs over the long-term, and allocate resources towards growth, reproduction, and immune function rather than fright responses alone [8,24,70,71]). We did not see differences in routine metabolic rate across exposure treatments in either the conditioned or unconditioned individuals. This may be because 30 min exposure periods were too short to elicit a physiological response among hellbenders or because hellbenders invest in physiological changes over chronic time periods rather than rapidly shifting metabolic responses over acute exposure events. Regardless, unconditioned individuals showed consistently higher oxygen consumption rates, leaving them at a physiological disadvantage, compared to conditioned individuals.
Our results suggest that predator conditioning can beneficially prepare hellbenders for release into the wild by strengthening their avoidance behaviors and promoting energy savings through physiological changes. Growth, behavioral avoidance, risk assessment, and the metabolic shifts that we observed among conditioned hellbenders are all evolutionary advantageous responses to predation risk [69]. Conditioned hellbenders elicited behavioral and physiological responses that reduce naïveté to predators, susceptibility to lethal attacks, sublethal effects, and additional stress during translocations and may ultimately improve the post-release survival and long-term persistence of wild hellbender populations. Future work will investigate the influence of predator conditioning on hellbender translocation success, as others have shown predator conditioning to improve survival. For example, white seabream (Diplodus sargus) are nearly twice as likely to survive following wild release if they are conditioned to conger eel (Conger conger; [72]). Furthermore, brook trout (Salvelinus fontinalus) have a 20% increase in survival during staged encounters with predatory pickerel following conditioning [73]. Animals often rely on previous encounters with predators to learn necessary avoidance strategies; however, these experiences can be stressful for and potentially lethal to prey [74]. Predator conditioning may effectively remove this dangerous learning period in the safety of a captive environment. Ultimately, predator conditioning enables animals to enter into risky environments with experiences and honed skills that will help them avoid predation. Future work can also explore conditioning techniques with other predators such as raccoons (Procyon lotor) or river otters (Lontra canidensis), which have been observed capturing and eating hellbenders in the wild [28]. Although we were able to account for genetic differentiation in our project by only using hellbenders from a single clutch of eggs, exploring the variation in predator responses within and among hellbender populations could also be beneficial. We found predator conditioning to be a low cost technique that required minimum amounts of time and effort to effectively induce behavioral and physiological changes among captive-reared hellbenders. Therefore, this method could be valuable to other imperiled vertebrates planned for translocation and at risk of wild predation. Captive-rearing programs should explore the potential for predator conditioning to prepare animals for wild release as this technique may have profound impacts on future translocation success.
5. Conclusions
Predator conditioning combats naïveté to predators and often results in prey having improved escape skills, appropriate avoidance behaviors, dampened fright responses, and higher survival during subsequent predator encounters. Our study substantiates claims that predator conditioning is advantageous to prey species and provides strong evidence that it prepares hellbenders for a wild environment with largemouth bass. Exposure during a 30 d conditioning period strengthened hellbenders’ behavioral avoidance skills and induced physiological changes that were absent from unconditioned individuals. Lower activity levels reduce hellbenders’ chances of encountering, being detected, or captured by predatory fish. Furthermore, lower metabolic rates allow conditioned hellbenders to conserve energy and balance the trade-off between predator avoidance and energy acquisition. Our data suggest that predator conditioning could improve future translocation efforts for hellbenders and for a multitude of imperiled vertebrate species.
Acknowledgments
We thank the Indiana Department of Natural Resources for funding this project (grant numbers T7R17 and T7R15). We are grateful to Todd Houser for his active involvement in construction, husbandry, and data collection. We thank Jason Hoverman for the use of his aquatic respirometer and Sam Gallagher for her instruction of and guidance with the system. We are also appreciative of Bob Rode for the use of his fish and making space available at Purdue University’s Aquaculture Research Laboratory as well as members of the Williams lab and Elizabeth Flaherty, William Hopkins, Jason Hoverman, and Catherine Searle for their constructive comments.
Author Contributions
Both authors contributed substantially to this project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Teixeira, C.P.; Schetini De Azevedo, C.; Mendl, M.; Cipreste, C.F.; Young, R.J. Revisiting translocation and reintroduction programmes : The importance of considering stress. Anim. Behav. 2007, 73, 1–13. [Google Scholar] [CrossRef]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress and translocation: Alterations in the stress physiology of translocated birds. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Delehanty, D.J.; Michael Romero, L. Stress: An inevitable component of animal translocation. Biol. Conserv. 2010, 143, 1329–1341. [Google Scholar] [CrossRef]
- Engelsma, M.Y.; Hougee, S.; Nap, D.; Hofenk, M.; Rombout, J.H.W.M.; Van Muiswinkel, W.B.; Lidy Verburg-van Kemenade, B.M. Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp, Cyprinus carpio L. Fish Shellfish Immunol. 2003, 15, 397–410. [Google Scholar] [CrossRef]
- Breves, J.P.; Specker, J.L. Cortisol stress response of juvenile winter flounder (Pseudopleuronectes americanus, Walbaum) to predators. J. Exp. Mar. Biol. Ecol. 2005, 325, 1–7. [Google Scholar] [CrossRef]
- Poledník, L.; Řehulka, J.; Kranz, A.; Poledníková, K.; Hlaváč, V.; Kazihnitkoví, H. Physiological responses of over-wintering common carp (Cyprinus carpio) to disturbance by Eurasian otter (Lutra lutra). Fish Physiol. Biochem. 2008, 34, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Brown, J.A. Energetic cost of reduced foraging under predation threat in newly hatched ocean pout. Mar. Ecol. Prog. Ser. 2006, 321, 255–266. [Google Scholar] [CrossRef]
- Van Dievel, M.; Janssens, L.; Stoks, R. Short- and long-term behavioural, physiological and stoichiometric responses to predation risk indicate chronic stress and compensatory mechanisms. Oecologia 2016, 181, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Aarts, L.; Van Schagen, I. Driving speed and the risk of road crashes: A review. Accid. Anal. Prev. 2006, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Chipman, R.; Slate, D.; Rupprecht, C.; Mendoza, M. Downside risk of wildlife translocation. Dev. Biol. (Basel) 2008, 131, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Crane, A.L.; Mathis, A. Predator-recognition training: A conservation strategy to increase postrelease survival of hellbenders in head-starting programs. Zoo Biol. 2011, 30, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Kellison, G.T.; Eggleston, D.B.; Burke, J.S. Comparative behaviour and survival of hatchery-reared versus wild summer flounder (Paralichthys dentatus). Can. J. Fish. Aquat. Sci. 2000, 57, 1870–1877. [Google Scholar] [CrossRef]
- Parish, D.M.B.; Sotherton, N.W. The fate of released captive-reared grey partridges Perdix perdix: Implications for reintroduction programmes. Wildl. Biol. 2007, 13, 140–149. [Google Scholar] [CrossRef]
- Pfeiffer, W. The fright reaction of fish. Biol. Rev. 1962, 37, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.E.; Anholt, B.R. Predator-induced behavioral indirect effects: Consequences to competitive interactions in anuran larvae. Ecology 1996, 77, 157–169. [Google Scholar] [CrossRef]
- Fraser, D.F.; Gilliam, J.F. Nonlethal impacts of predator invasion: Facultative suppression of growth and reproduction. Ecology 1992, 73, 959–970. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Endocrinology of the stress-response. In Behavioral Endocrinology; Becker, J.B., Breedlove, S.M., Crews, D., McCarthy, M.M., Eds.; MIT Press: Cambridge, UK, 2002; pp. 409–450. [Google Scholar]
- Slos, S.; Stoks, R. Predation risk induces stress proteins and reduces antioxidant defense. Funct. Ecol. 2008, 22, 637–642. [Google Scholar] [CrossRef]
- Steiner, U.K.; Van Buskirk, J. Predator-induced changes in metabolism cannot explain the growth/predation risk tradeoff. PLoS ONE 2009, 4, e6160. [Google Scholar] [CrossRef] [PubMed]
- Lankford, S.E.; Adams, T.E.; Miller, R.A.; Cech, J.J., Jr. The cost of chronic stress: Impacts of a nonhabituating stress response on metabolic variables and swimming performance in sturgeon. Physiol. Biochem. Zool. 2005, 78, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, H.F.; Bodie, B.L.; Tauchi, M.; Dolgas, C.M.; Herman, J.P. Stress integration after acute and chronic predator stress: Differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology 2003, 144, 5249–5258. [Google Scholar] [CrossRef] [PubMed]
- Petersson, E.; Valencia, A.C.; Järvi, T. Failure of predator conditioning: An experimental study of predator avoidance in brown trout (Salmo trutta). Ecol. Freshw. Fish 2015, 24, 329–337. [Google Scholar] [CrossRef]
- Chivers, D.P.; Mirza, R.S. Importance of predator diet cues in responses of larval wood frogs to fish and invertebrate predators. J. Chem. Ecol. 2001, 27, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Gardner, C.; Braithwaite, V.A. Differential stress responses in fish from areas of high- and low-predation pressure. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2005, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.J.; Smith, L.S. Exercise training and the stress response in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1985, 26, 435–447. [Google Scholar] [CrossRef]
- Young, P.S.; Cech, J.J.J. Effects of exercise conditioning on stress response and recovery in cultured and wild young-of-the-year striped bass, Morone saxatilis. Can. J. Fish. Aquat. Sci. 1993, 50, 2094–2099. [Google Scholar] [CrossRef]
- Bodinof, C.M.; Briggler, J.T.; Junge, R.E.; Mong, T.; Beringer, J.; Wanner, M.D.; Schuette, C.D.; Ettling, J.; Millspaugh, J.J. Survival and body condition of captive-reared juvenile Ozark hellbenders (Cryptobranchus alleganiensis bishopi) following translocation to the wild. Copeia 2012, 2012, 150–159. [Google Scholar] [CrossRef]
- Boerner, J.A. Comparison of Movement Patterns in Captive-Released Eastern Hellbenders (Cryptobranchus alleganiensis alleganiensis) Using Three Different Release Methods; State University of New York Buffalo: Buffalo, NY, USA, 2014. [Google Scholar]
- Kraus, B.T.; McCallen, E.B.; Williams, R.N. Evaluating the survival of translocated adult and captive-reared, juvenile eastern hellbenders (Cryptobranchus alleganiensis alleganiensis). Herpetologica 2017, 73, 271–276. [Google Scholar] [CrossRef]
- Carnahan, D.P. Blue River Fisheries Survey and Game Fish Population Estimates in Crawford, Harrison, and Washington Counties; Indiana Department of Natural Resources: Indianapolis, Indiana, 2001. [Google Scholar]
- Gall, B.G. Predator-Prey Interactions between Hellbenders (Cryptobranchus alleganiensis alleganiensis) and (CA. Bishopi) and Native and Nonnative Fishes. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2008. [Google Scholar]
- Gall, B.G.; Mathis, A. Innate predator recognition and the problem of introduced trout. Ethology 2010, 116, 47–58. [Google Scholar] [CrossRef]
- Burgmeier, N.G.; Unger, S.D.; Sutton, T.M.; Williams, R.N. Population status of the eastern hellbender (Cryptobranchus alleganiensis alleganiensis) in Indiana. J. Herpetol. 2011, 45, 195–201. [Google Scholar] [CrossRef]
- Kats, L.B.; Petranka, J.W.; Sih, A. Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 1988, 69, 1865–1870. [Google Scholar] [CrossRef]
- Barbosa, P.; Castellanos, I. Ecology of Predator-Prey Interactions; Oxford University Press: Oxford, UK, 2005; ISBN 9780195171204. [Google Scholar]
- Ferland-Raymond, B.; Murray, D.L. Predator diet and prey adaptive responses: Can tadpoles distinguish between predators feeding on congeneric vs. conspecific prey? Can. J. Zool. 2008, 86, 1329–1336. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. When should prey respond to consumed heterospecifics? Testing hypotheses of perceived risk. Copeia 2009, 2009, 190–194. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Maher, J.M.; Werner, E.E.; Denver, R.J. Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc. R. Soc. B Biol. Sci. 2013, 280, 1–9. [Google Scholar] [CrossRef]
- Hall, A.E.; Clark, T.D. Seeing is believing: Metabolism provides insight into threat perception for a prey species of coral reef fish. Anim. Behav. 2016, 115, 117–126. [Google Scholar] [CrossRef]
- Burraco, P.; Duarte, L.J.; Gomez-Mestre, I. Predator-induced physiological responses in tadpoles challenged with herbicide pollution. Curr. Zool. 2013, 59, 475–484. [Google Scholar] [CrossRef]
- Alvarez, D.; Cano, J.M.; Nicieza, A.G. Microgeographic variation in metabolic rate and energy storage of brown trout: Countergradient selection or thermal sensitivity? Evol. Ecol. 2006, 20, 345–363. [Google Scholar] [CrossRef]
- Relyea, R.A. Fine-tuned phenotypes: Tadpole plasticity under 16 combinations of predators and competitors. Ecology 2004, 85, 172–179. [Google Scholar] [CrossRef]
- Orlofske, S.A.; Hopkins, W.A. Energetics of metamorphic climax in the pickerel frog (Lithobates palustris). Comp. Biochem. Physiol. Part A 2009, 154, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kearney, B.D.; Byrne, P.G.; Reina, R.D. Short-and long-term consequences of developmental saline stress: Impacts on anuran respiration and behaviour. R. Soc. Open Sci. 2016, 3, 150640. [Google Scholar] [CrossRef] [PubMed]
- Srean, P.; Almeida, D.; Rubio-Gracia, F.; Luo, Y.; Garcia-Berthou, E. Effects of size and sex on swimming performance and metabolism of invasive mosquitofish Gambusia holbrooki. Ecol. Freshw. Fish 2017, 26, 424–433. [Google Scholar] [CrossRef]
- Guimond, R.W.; Hutchison, V.H. Aquatic respiration: An unusual strategy in the hellbender Cryptobranchus alleganiensis alleganiensis (Daudin). Science 1973, 182, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Conover, W.J. Practical Nonparametric Statistics; Wiley: Hoboken, NJ, USA, 1999; ISBN 0471160687. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Norrdahl, K.; Korpimäki, E. Fear in farmlands: How much does predator avoidance affect bird community structure? J. Avian Biol. 1998, 29, 79–85. [Google Scholar] [CrossRef]
- Kenison, E.K.E.K.; Weldy, P.Y.P.Y.; Williams, R.N.R.N. There must be something in the water: Assessing the behavioral responses of rusty crayfish (Orconectes rusticus) to fish and amphibian predator kairomones. J. Ethol. 2017, 1–8. [Google Scholar] [CrossRef]
- Bleckmann, H.; Zelick, R. Lateral line system of fish. Integr. Zool. 2009, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- McPeek, M.A. The growth/predation risk trade-off: So what is the mechanism? Am. Nat. 2004, 163, E88–E111. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, I.J.; Aho, J.; Vornanen, M.; Huuskonen, H. Phenotypic plasticity and predator effects on morphology and physiology of crucian carp in nature and in the laboratory. J. Fish Biol. 1997, 50, 781–798. [Google Scholar] [CrossRef]
- Brönmark, C.; Miner, J.G. Predator-induced phenotypical change in body morphology in crucian carp. Science 1992, 258, 1348–1350. [Google Scholar] [CrossRef] [PubMed]
- Repka, S.; Walls, M.; Kettle, M. Neck spine protects Daphnia Pulex from predation by Chaoborus, but individuals with longer tail spine are at a greater risk. J. Plankton Res. 1995, 17, 393–403. [Google Scholar] [CrossRef]
- Hoverman, J.T.; Auld, J.R.; Relyea, R.A. Putting prey back together again: Integrating predator-induced behavior, morphology, and life history. Oecologia 2005, 144, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Haskell, A.; Graham, T.E.; Griffin, C.R.; Hestbeck, J.B. Size related survival of headstarted redbelly turtles (Pseudemys rubriventris) in Massachusetts. J. Herpetol. 1996, 30, 524–527. [Google Scholar] [CrossRef]
- Kats, L.B.; Dill, L.M. The scent of death: Chemosensory assessment of predation risk by prey animals. Écoscience 1998, 5, 361–394. [Google Scholar] [CrossRef]
- Epp, K.J.; Gabor, C.R. Innate and learned predator recognition mediated by chemical signals in Eurycea nana. Ethology 2008, 114, 607–615. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Damage, digestion, and defence: The roles of alarm cues and kairomones for inducing prey defences. Ecol. Lett. 2005, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Cost and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Relyea, R.A. Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol. J. Linn. Soc. 1998, 65, 301–328. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Interpreting the smells of predation: How alarm cues and kairomones induce different prey defences. Funct. Ecol. 2009, 23, 1114–1121. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Detecting small environmental differences: Risk-response curves for predator-induced behavior and morphology. Oecologia 2008, 154, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Van Buskirk, J.; Muller, C.; Portmann, A.; Surbeck, M. A test of the risk allocation hypothesis: Tadpole responses to temporal change in predation risk. Behav. Ecol. 2002, 13, 526–530. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef]
- Sih, A.; McCarthy, T.M. Prey responses to pulses of risk and safety: Testing the risk allocation hypothesis. Anim. Behav. 2002, 63, 437–443. [Google Scholar] [CrossRef]
- Barry, M.J.; Syal, S. Metabolic responses of tadpoles to chemical predation cues. Hydrobiologia 2013, 700, 267–276. [Google Scholar] [CrossRef]
- Reed, D.H.; Lowe, E.H.; Briscoe, D.A.; Frankham, R. Fitness and adaptation in a novel environment: Effect of inbreeding, prior environment, and lineage. Evolution (N. Y.) 2003, 57, 1822–1828. [Google Scholar] [CrossRef]
- Handelsman, C.A.; Broder, E.D.; Dalton, C.M.; Ruell, E.W.; Myrick, C.A.; Reznick, D.N.; Ghalambor, C.K. Predator-induced phenotypic plasticity in metabolism and rate of growth: Rapid adaptation to a novel environment. Integr. Comp. Biol. 2013, 53, 975–988. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, G.; Giacalone, V.M.; Vega Fernández, T.; Vaccaro, A.M.; Pipitone, C.; Mirto, S.; Mazzola, S.; Badalamenti, F. Effects of predator and shelter conditioning on hatchery-reared white seabream Diplodus sargus (L., 1758) released at sea. Aquaculture 2012, 356–357, 91–97. [Google Scholar] [CrossRef]
- Mirza, R.S.; Chivers, D.P. Predator-recognition training enhances survival of brook trout: Evidence from laboratory and field- enclosure studies. Can. J. Zool. 2000, 2208, 2198–2208. [Google Scholar] [CrossRef]
- Griffin, A.S.; Blumstein, D.T.; Evans, C.S. Review: Training captive-bred or translocated animals to avoid predators. Conserv. Biol. 2000, 14, 1317–1326. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
