Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Data Extraction
2.3. Endpoint Definition
2.4. Low-Level Light/Laser Therapy (LLLT) Protocols and Devices
- Blue light (Figure 1C), with a wavelength of 423 nm and a skin penetration depth of 1 mm, activates the keratin present in the hair shaft, increases the water retention of hair, acts in the microbiological control, and diminishes the sebaceous gland, reducing the grease of the scalp;
- Red light (Figure 1D), with a wavelength of 640 nm and a skin penetration depth of 1–6 mm, promotes cell metabolism, improves blood circulation, promotes nutrition supplies to capillaries, strengthens hair strands, promotes the alignment of the cuticles, and promotes pain relief.
2.5. Patients
2.6. The Risk Mitigation Measures
- -
- The informed consent for all patients, where the risks (represented only by ineffective results) and side effects of the procedures were reported;
- -
- A specific training plan to healthcare providers;
- -
- A communications plan of the side effects (represented only by itching, slight redness, slight numbness of the treated area, and headaches);
- -
- The need for CE marks for the medical devices used; and
- -
- The need to enroll patients through the inclusion and exclusion criteria.
2.7. The Trichoscopy Evaluation of the Targeted Area
2.8. Statistical Analysis
3. Results
3.1. Instrumental Evaluation Using Trichoscopy Analysis
3.2. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alsantali, A.; Shapiro, J. Androgens and hair loss. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef]
- Gentile, P.; Cole, J.P.; Cole, M.A.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Insalaco, C.; Cervelli, V. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int. J. Mol. Sci. 2017, 18, 408. [Google Scholar] [CrossRef] [PubMed]
- Mester, E.; Szende, B.; Tota, J. Effect of laser on hair growth of mice. Kiserl Orv. 1967, 19, 628–631. [Google Scholar]
- Hamblin, M.R. Photobiomodulation for the management of alopecia: Mechanisms of action, patient selection, and perspectives. Clin. Cosmet. Investig. Derm. 2019, 12, 669. [Google Scholar] [CrossRef] [Green Version]
- Wikramanayake, T.C.; Rodriguez, R.; Choudhary, S.; Mauro, L.M.; Nouri, K.; Schachner, L.A.; Jimenez, J.J. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med. Sci. 2012, 27, 431–436. [Google Scholar] [CrossRef]
- Kim, W.S.; Calderhead, R.G. Is light-emitting diode phototherapy (LED-LLLT) effective? Laser Ther. 2011, 20, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci, P.; Gupta, G.K.; Clark, J.; Wikonkal, N.; Hamblin, M.R. Low-Level Laser (Light) Therapy (LLLT) for Treatment of Hair Loss. Lasers Surg. Med. 2013, 46, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Suchonwanit, P.; Rojhirunsakool, S.; Khunkhet, S. A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia. Lasers Med. Sci. 2019, 34, 1857–1864. [Google Scholar] [CrossRef]
- Ash, C.; Harrison, A.; Drew, S.; Whittall, R. A randomized controlled study for the treatment of acne vulgaris using high-intensity 414 nm solid-state diode arrays. J. Cosmet Laser Ther. 2015, 4, 170–176. [Google Scholar] [CrossRef]
- Naranjo García, P.; Elias, J.A.; Gaviria Parada, J.; Zarza Luciañez, D.; Pinto, H.R. Management of Vaginal Atrophy with Intravaginal Light-Emitting Diodes (LEDs). Int J. Obstet. Gyanecol. Res. 2018, 5, 632–641. [Google Scholar]
- Calderhead, R.G.; Vasily, D.B. Low-Level Light Therapy with Light-Emitting Diodes for the Aging Face. Clin. Plast. Surg. 2016, 43, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.A.; McDaniel, D.H.; Geronemus, R.G.; Weiss, M.A. Clinical trial of a novel non-thermal LED array for reversal of photoaging: Clinical, histologic, and surface profilometric results. Lasers Surg. Med. 2005, 36, 85–91. [Google Scholar] [CrossRef]
- Yang, K.; Tang, Y.; Ma, Y.; Liu, Q.; Huang, Y.; Zhang, Y.; Shi, X.; Zhang, L.; Zhang, Y.; Wang, J.; et al. Hair Growth Promoting Effects of 650 nm Red Light Stimulation on Human Hair Follicles and Study of Its Mechanisms via RNA Sequencing Transcriptome Analysis. Ann. Dermatol. 2021, 33, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Dionisi, L.; Pizzicannella, J.; de Angelis, B.; de Fazio, D.; Garcovich, S. A randomized blinded retrospective study: The combined use of micro-needling technique, low-level laser therapy, and autologous non-activated platelet-rich plasma improves hair re-growth in patients with androgenic alopecia. Exp. Opin. Biol. Ther. 2020, 20, 1099–1109. [Google Scholar] [CrossRef]
- Schuklenk, U.; Ashcroft, R. International research ethics. Bioethics 2000, 14, 158–172. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Gentile, P.; Garcovich, S. The Effectiveness of Low-Level Light/Laser Therapy on Hair Loss. Facial. Plast. Surg. Aesthet. Med. 2021. [Google Scholar] [CrossRef]
- Mahe, Y.F.; Cheniti, A.; Tacheau, C.; Antonelli, R.; Planard-Luong, L.; de Bernard, S.; Buffat, L.; Barbarat, P.; Kanoun-Copy, L. Low-Level Light Therapy Downregulates Scalp Inflammatory Biomarkers in Men With Androgenetic Alopecia and Boosts Minoxidil 2% to Bring a Sustainable Hair Regrowth Activity. Lasers Surg. Med. 2021, 53, 1208–1219. [Google Scholar] [CrossRef]
- Darwin, E.; Heyes, A.; Hirt, P.A.; Wikramanayake, T.C.; Jimenez, J.J. Low-level laser therapy for the treatment of androgenic alopecia: A review. Lasers Med. Sci. 2018, 21, 425–434. [Google Scholar] [CrossRef]
- Barikbin, B.; Khodamrdi, Z.; Kholoosi, L.; Akhgri, M.R.; Abbasi, M.H.; Hajabbasi, M.; Razzaghi, Z.; Akbarpour, S. Comparison of the effects of 665 nm low-level diode Laser Hat versus and a combination of 665 nm and 808nm low-level diode Laser Scanner of hair growth in androgenic alopecia. J. Cosmet. Laser Ther. 2017, 17. [Google Scholar] [CrossRef]
- Afifi, L.; Maranda, E.L.; Zarei, M.; Delcanto, G.M.; Falto-Aizpurua, L.; Kluijfhout, W.P.; Jimenez, J.J. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg. Med. 2017, 49, 27–39. [Google Scholar] [CrossRef]
- Avram, M.R.; Rogers, N.E. The use of low-level light for hair growth: Part I. J. Cosmet Laser Ther. 2009, 12, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Jeong, K.H.; Kim, J.E.; Kang, H. Various Wavelengths of Light-Emitting Diode Light Regulate the Proliferation of Human Dermal Papilla Cells and Hair Follicles via Wnt/B-Catenin and the Extracellular Signal-Regulated Kinase Pathways. Ann. Dermatol. 2017, 29, 747. [Google Scholar] [CrossRef] [PubMed]
- Sorbellini, E.; Rucco, M.; Rinaldi, F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: An update. Lasers Med. Sci. 2018, 33, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Leavitt, M.; Charles, G.; Heyman, E.; Michaels, D. HairMax LaserComb® laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin. Drug Investig. 2009, 29, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Suchonwanit, P.; Chalermroj, N.; Khunkhet, S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: A 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med. Sci. 2018, 19, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Friedman, S.; Schnoor, P. Novel approach to treating androgenetic alopecia in females with photobiomodulation (low-level laser therapy). Derm. Surg. 2017, 43, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Lanzafame, R.J.; Blanche, R.R.; Chiacchierini, R.P.; Kazmirek, E.R.; Sklar, J.A. The growth of human scalp hair in females using visible red-light laser and LED sources. Lasers Surg. Med. 2014, 46, 601–607. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Wikramanayake, T.C.; Bergfeld, W.; Hordinsky, M.; Hickman, J.G.; Hamblin, M.R.; Schachner, L.A. Efficacy and safety of a low-level laser device in the treatment of male and female pattern hair loss: A multicenter, randomized, sham device-controlled, double-blind study. Am. J. Clin. Dermatol. 2014, 15, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Lanzafame, R.J.; Blanche, R.R.; Bodian, A.B.; Chiacchierini, R.P.; Fernandez-Obregon, A.; Kazmirek, E.R. The growth of human scalp hair mediated by visible red-light laser and LED sources in males. Lasers Surg. Med. 2013, 45, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.W.; Kim, J.Y.; Shin, J.W.; Lee, S.-J.; Huh, C.-H. Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Derm. Surg. 2013, 39, 1177–1183. [Google Scholar] [CrossRef]
- Fan, S.M.-Y.; Cheng, Y.-P.; Lee, M.-Y.; Lin, S.-J.; Chiu, H.-Y. Efficacy and Safety of a Low-Level Light Therapy for Androgenetic Alopecia: A 24-Week, Randomized, Double-Blind, Self-Comparison, Sham Device-Controlled Trial. Derm. Surg. 2018, 44, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Satino, J.L.; Markou, M. Hair regrowth and increased hair tensile strength using the HairMax LaserComb for low-level laser therapy. Int J. Cosmet Surg. Aesthetic Dermatol. 2003, 5, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Munck, A.; Gavazzoni, M.F.; Trüeb, R.M. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int. J. Trichology 2014, 6, 45. [Google Scholar]
- Esmat, S.M.; Hegazy, R.A.; Gawdat, H.I.; Hay, R.A.; Allam, R.; El Naggar, R.; Moneib, H. Low-level light-minoxidil 5% combination versus either therapeutic modality alone in the management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med. 2017, 49, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light-emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
- Mosca, R.C.; Ong, A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Ski. Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I.; Kalendo, G.S.; Letokhov, V.S.; Lobko, V.V. Biostimulation of HeLa cells by low-intensity visible light. Il Nuovo Cim. D 1982, 1, 828–840. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, A.C.-H.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low-level light therapy. Dose-Response 2009, 7, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.L.; Fiedler, V.; Imperato-McGinley, J.; Whiting, D.; Olsen, E.; Shupack, J.; Stough, D.; DeVillez, R.; Rietschel, R.; Savin, R.; et al. Clinical dose-ranging studies with finasteride, a type 2 5α-reductase inhibitor, in men with male pattern hair loss. J. Am. Acad. Derm. 1999, 41, 555–563. [Google Scholar] [PubMed]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Derm. 2002, 47, 377–385. [Google Scholar] [CrossRef] [PubMed]

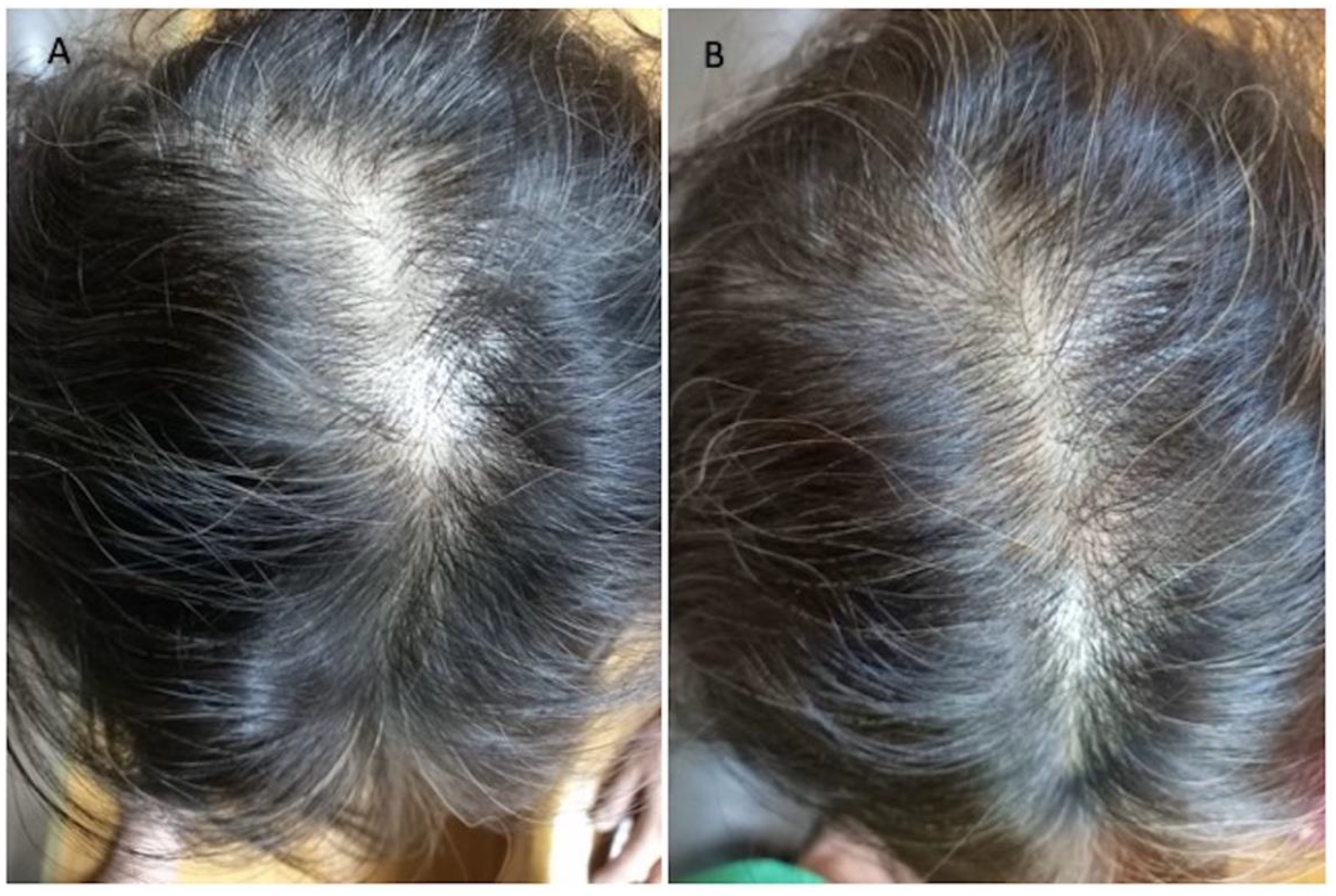
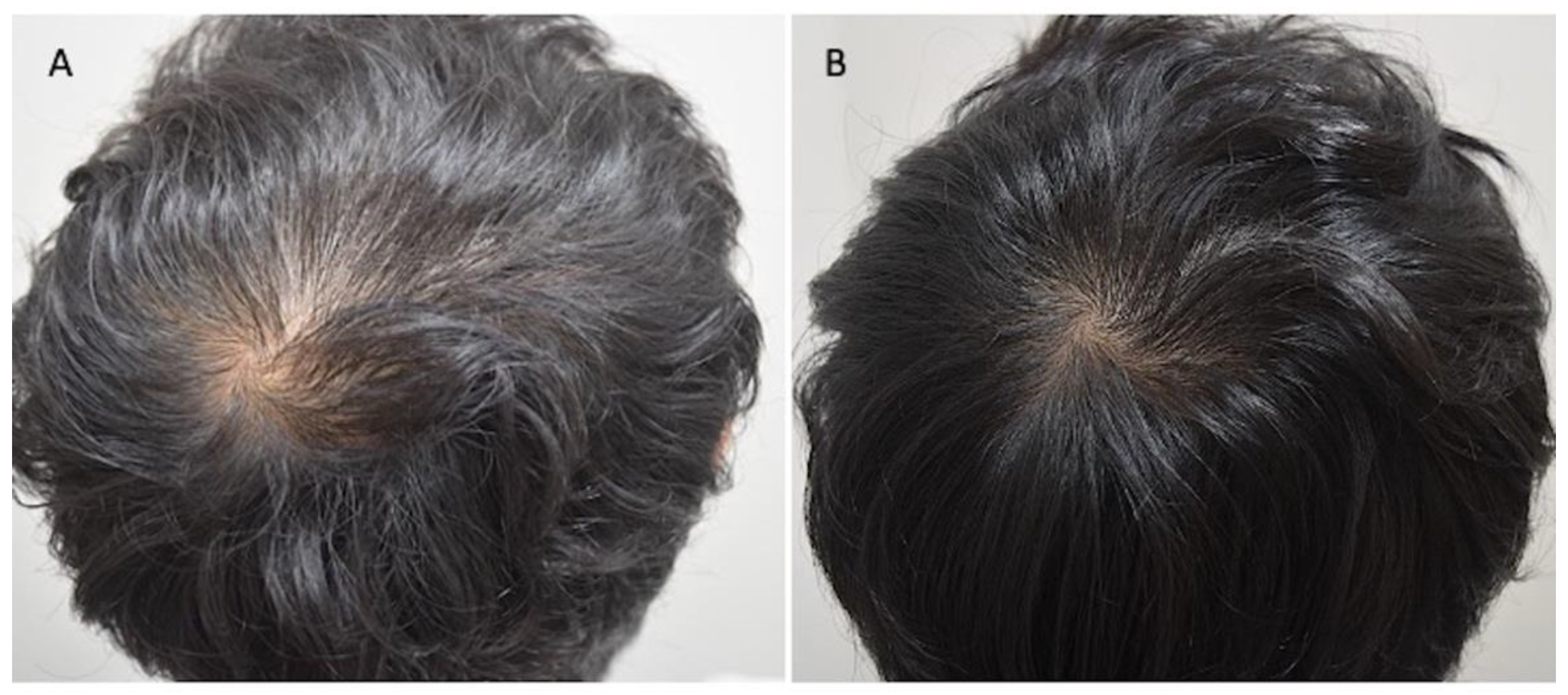
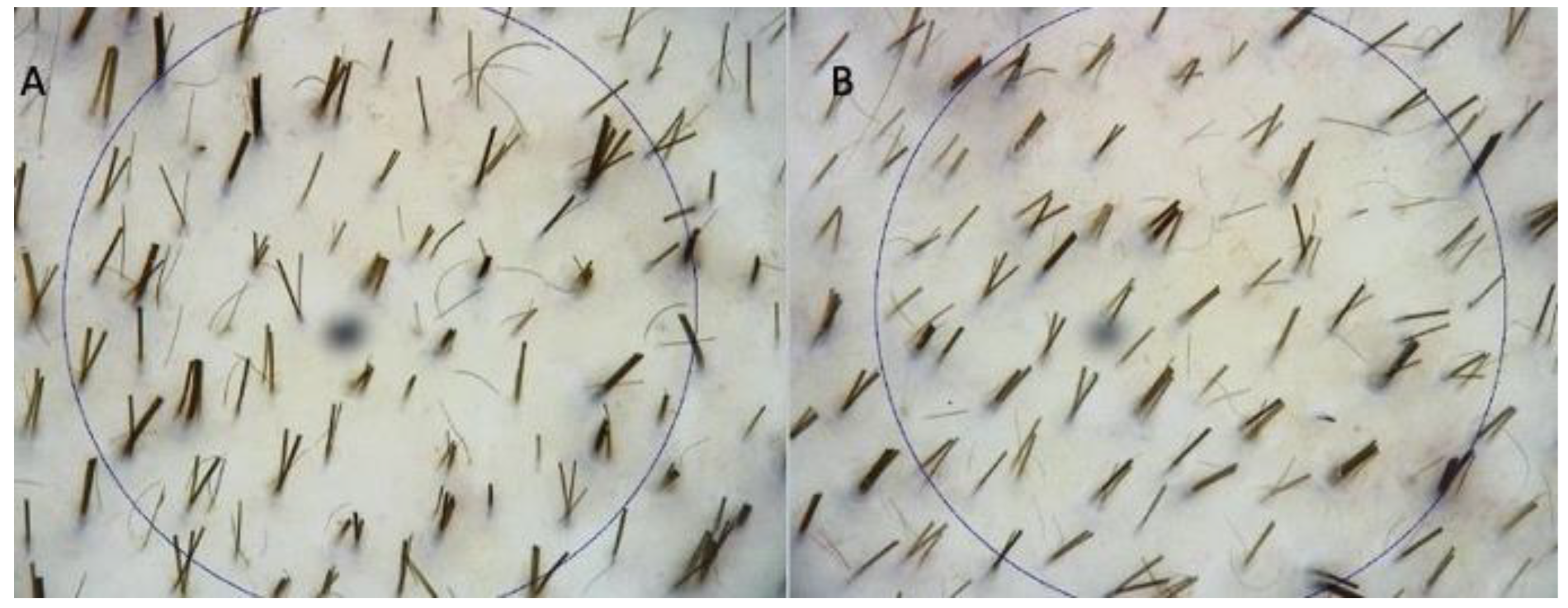
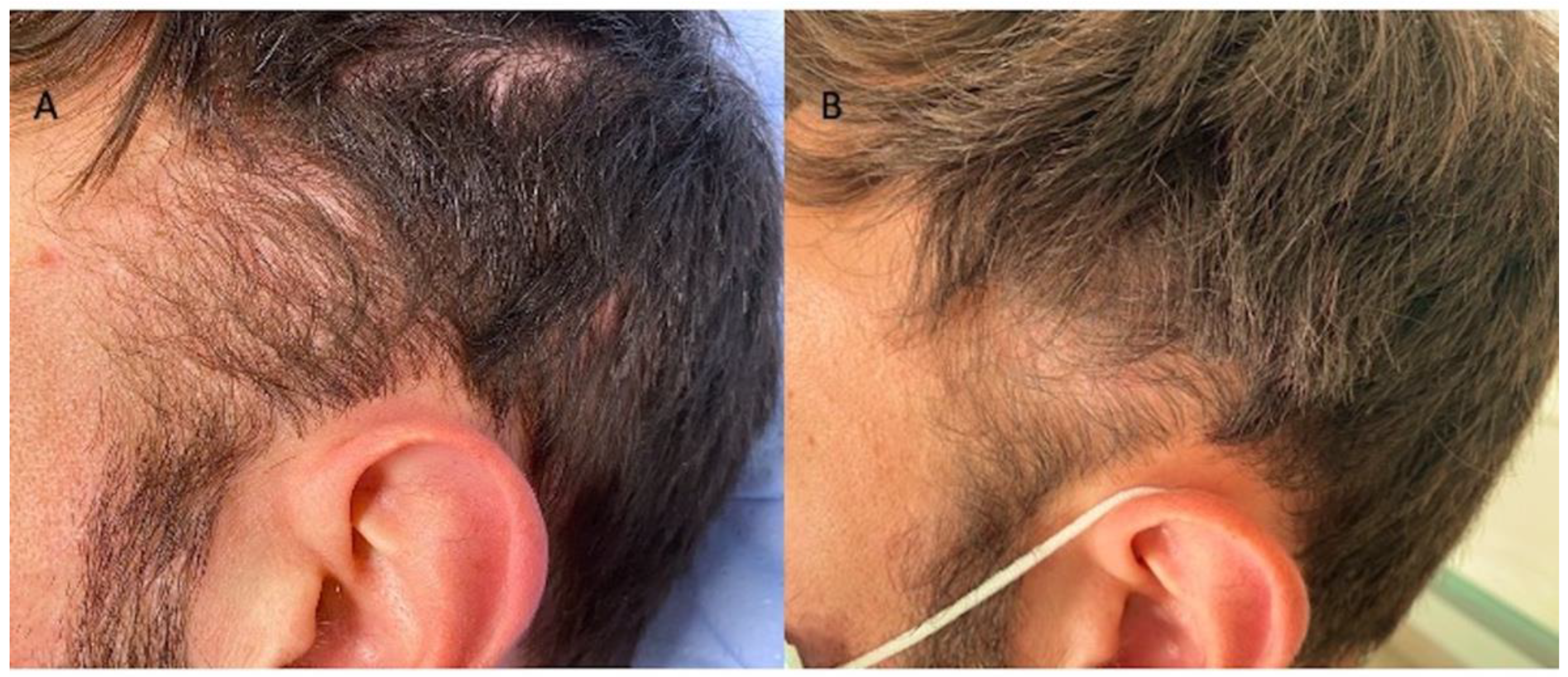
| Patients | Gender | Norwood–Hamilton Degree | Ludwig Degree | Targeted Area | Age | Race |
|---|---|---|---|---|---|---|
| 1 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 49 | Caucasian |
| 2 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 31 | Asian |
| 3 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 61 | Caucasian |
| 4 | Male | II | - | Frontal, temporal | 26 | Asian |
| 5 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 71 | Caucasian |
| 6 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 56 | Asian |
| 7 | Male | IIa | - | Frontal, temporal, parietal | 45 | Caucasian |
| 8 | Male | III-vertex | - | Frontal, temporal, parietal, vertex | 29 | Asian |
| 9 | Male | II | - | Frontal, temporal, parietal | 35 | Caucasian |
| 10 | Male | I | - | Frontal | 20 | Asian |
| 11 | Female | - | I | Frontal, parietal | 40 | Caucasian |
| 12 | Female | - | II | Frontal, temporal, parietal | 60 | Asian |
| 13 | Female | - | II | Frontal, temporal, parietal, vertex | 63 | Caucasian |
| 14 | Female | - | II | Frontal, temporal, parietal, vertex | 40 | Asian |
| 15 | Female | - | II | Frontal, temporal, parietal, vertex | 55 | Caucasian |
| 16 | Female | - | II | Frontal, temporal, parietal | 39 | Asian |
| 17 | Female | - | II | Frontal, temporal, parietal, vertex | 61 | Caucasian |
| 18 | Female | - | II | Frontal, temporal, parietal | 26 | Asian |
| 19 | Female | - | I | Frontal, parietal | 38 | Caucasian |
| 20 | Female | - | I | Frontal, parietal | 25 | Asian |
| Patients | Procedure | Hair Density (T0) | Hair Density (T1-16 wks) |
|---|---|---|---|
| 1 | DTSMG MTS stamp + HR3 matrix | 30 ± 2 hairs/cm2 | 42 ± 2 hairs/cm2 |
| 2 | DTSMG MTS stamp + HR3 matrix | 44 ± 2 hairs/cm2 | 55 ± 2 hairs/cm2 |
| 3 | DTSMG MTS stamp + HR3 matrix | 59 ± 2 hairs/cm2 | 78 ± 2 hairs/cm2 |
| 4 | DTSMG MTS stamp + HR3 matrix | 36 ± 2 hairs/cm2 | 45 ± 2 hairs/cm2 |
| 5 | DTSMG MTS stamp + HR3 matrix | 75 ± 2 hairs/cm2 | 96 ± 2 hairs/cm2 |
| 6 | DTSMG MTS stamp + HR3 matrix | 43 ± 2 hairs/cm2 | 52 ± 2 hairs/cm2 |
| 7 | DTSMG MTS stamp + HR3 matrix | 76 ± 2 hairs/cm2 | 97 ± 2 hairs/cm2 |
| 8 | DTSMG MTS stamp + HR3 matrix | 52 ± 2 hairs/cm2 | 60 ± 2 hairs/cm2 |
| 9 | DTSMG MTS stamp + HR3 matrix | 42 ± 2 hairs/cm2 | 60 ± 2 hairs/cm2 |
| 10 | DTSMG MTS stamp + HR3 matrix | 38 ± 2 hairs/cm2 | 44 ± 2 hairs/cm2 |
| 11 | DTSMG MTS stamp + HR3 matrix | 40 ± 2 hairs/cm2 | 53 ± 2 hairs/cm2 |
| 12 | DTSMG MTS stamp + HR3 matrix | 30 ± 2 hairs/cm2 | 42 ± 2 hairs/cm2 |
| 13 | DTSMG MTS stamp + HR3 matrix | 63 ± 2 hairs/cm2 | 80 ± 2 hairs/cm2 |
| 14 | DTSMG MTS stamp + HR3 matrix | 35 ± 2 hairs/cm2 | 41 ± 2 hairs/cm2 |
| 15 | DTSMG MTS stamp + HR3 matrix | 50 ± 2 hairs/cm2 | 65 ± 2 hairs/cm2 |
| 16 | DTSMG MTS stamp + HR3 matrix | 40 ± 2 hairs/cm2 | 44 ± 2 hairs/cm2 |
| 17 | DTSMG MTS stamp + HR3 matrix | 65 ± 2 hairs/cm2 | 78 ± 2 hairs/cm2 |
| 18 | DTSMG MTS stamp + HR3 matrix | 33 ± 2 hairs/cm2 | 38 ± 2 hairs/cm2 |
| 19 | DTSMG MTS stamp + HR3 matrix | 56 ± 2 hairs/cm2 | 65 ± 2 hairs/cm2 |
| 20 | DTSMG MTS stamp + HR3 matrix | 45 ± 2 hairs/cm2 | 57 ± 2 hairs/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentile, P.; Garcovich, S.; Lee, S.-I.; Han, S. Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia. Appl. Sci. 2022, 12, 217. https://doi.org/10.3390/app12010217
Gentile P, Garcovich S, Lee S-I, Han S. Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia. Applied Sciences. 2022; 12(1):217. https://doi.org/10.3390/app12010217
Chicago/Turabian StyleGentile, Pietro, Simone Garcovich, Soo-Ik Lee, and Sangbum Han. 2022. "Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia" Applied Sciences 12, no. 1: 217. https://doi.org/10.3390/app12010217
APA StyleGentile, P., Garcovich, S., Lee, S.-I., & Han, S. (2022). Regenerative Biotechnologies in Plastic Surgery: A Multicentric, Retrospective, Case-Series Study on the Use of Micro-Needling with Low-Level Light/Laser Therapy as a Hair Growth Boost in Patients Affected by Androgenetic Alopecia. Applied Sciences, 12(1), 217. https://doi.org/10.3390/app12010217







