Mechanisms of Melatonin in Obesity: A Review
Abstract
:1. Introduction
2. Effect of Melatonin on Obesity
2.1. Body Weight
2.2. Lipid Profile
2.3. Glucose Metabolism
2.4. Insulin Resistance
2.5. Prenatal Melatonin in Childhood Obesity
3. Effect of Melatonin on Obesity
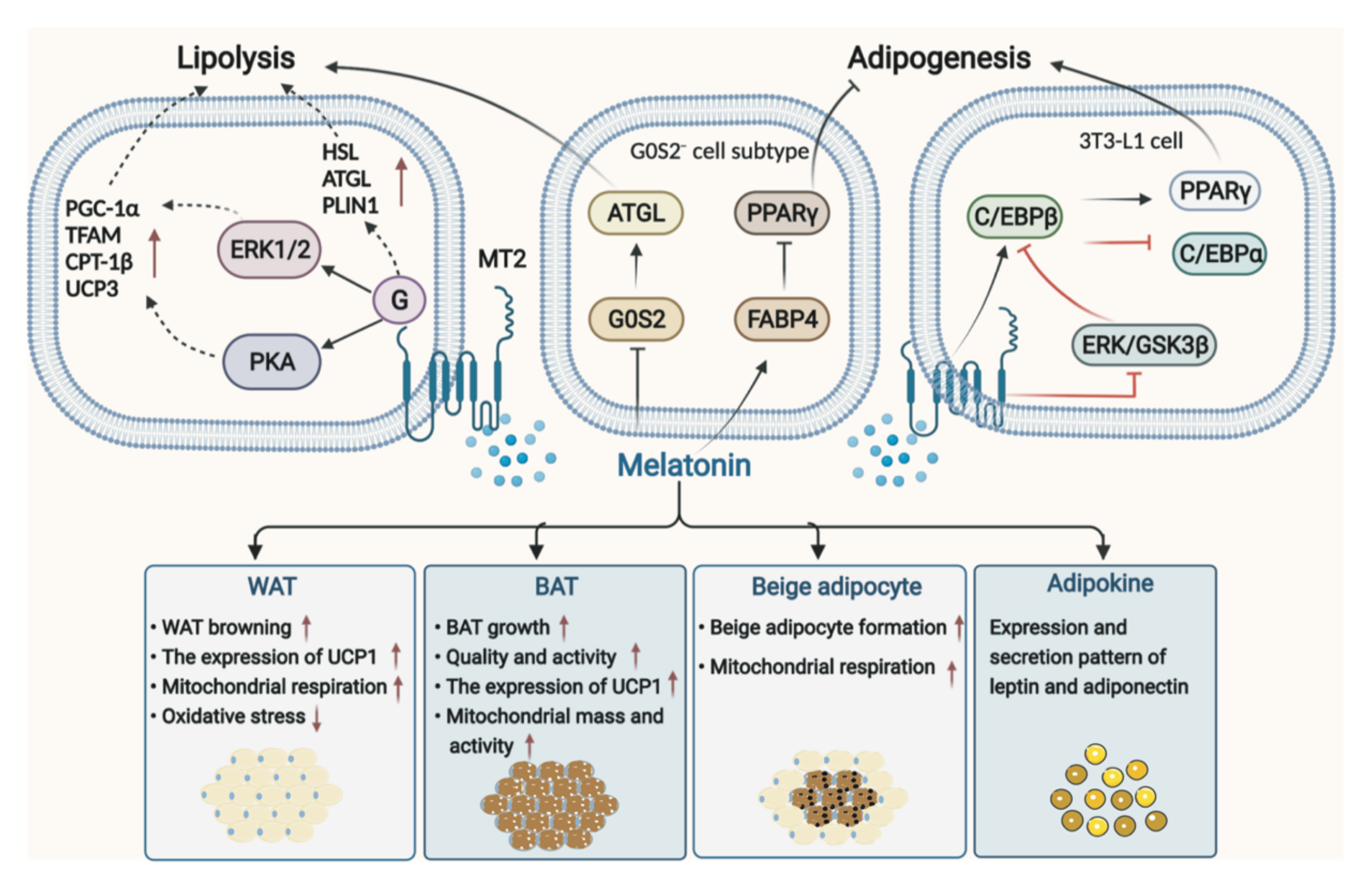
3.1. Melatonin in Adipose Tissue
3.1.1. Melatonin in WAT
3.1.2. Melatonin in BAT
3.1.3. Melatonin in Adipokines
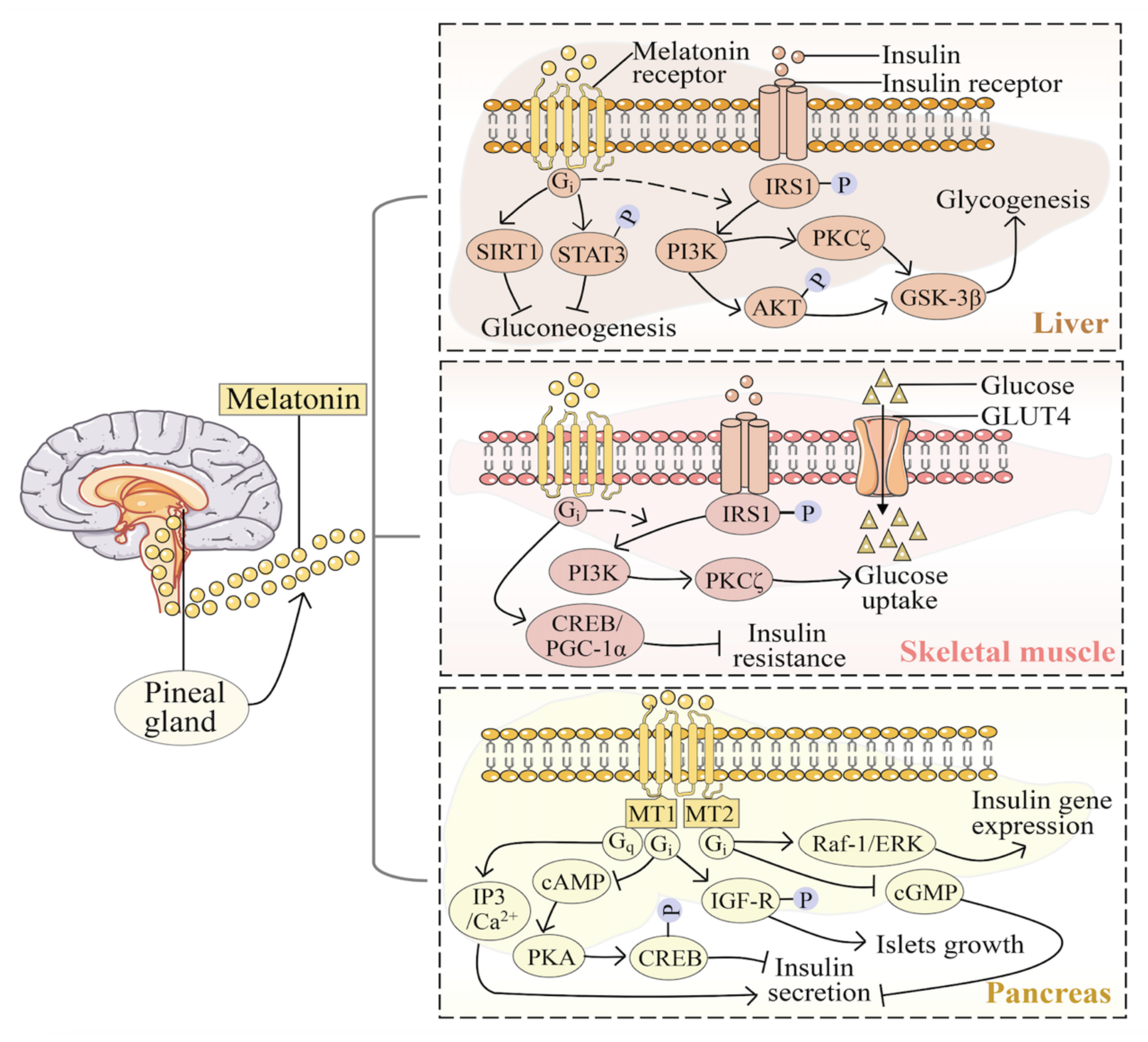
3.2. Melatonin in Liver
3.3. Melatonin in the Pancreas
3.4. Melatonin in Skeletal Muscle
4. Potential Mechanisms of Melatonin in Obesity
4.1. Melatonin Receptors
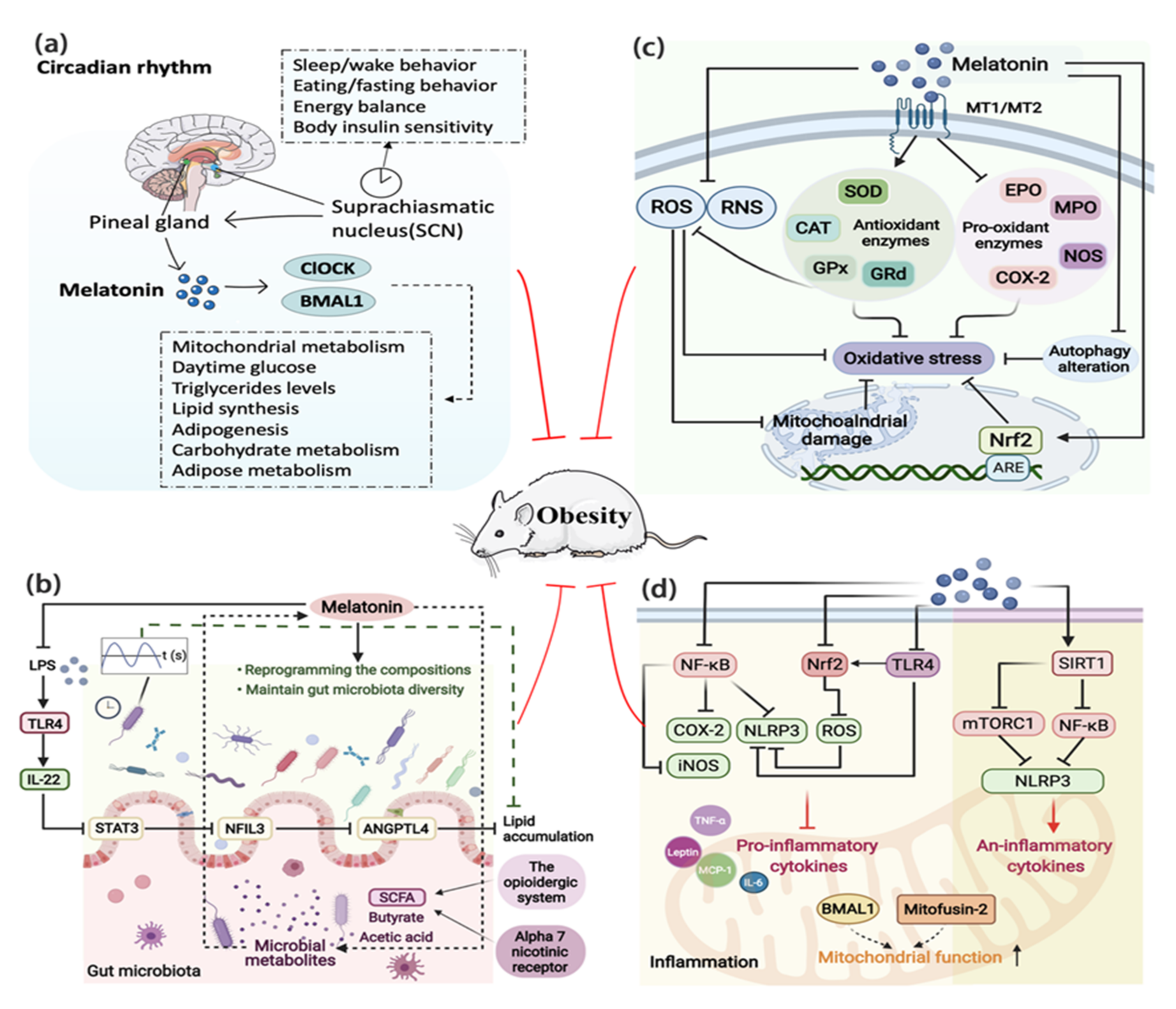
4.2. Circadian Rhythm
4.3. Involvement of the Gut Microbiota
4.4. Melatonin and Sleep Disorders
4.5. Melatonin and Oxidative Stress
4.6. Melatonin and Inflammation
4.7. Other Related Ways
5. Clinical Safety of Melatonin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mg, N.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-activated protein kinase (MAPK) and obesity-related cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system, and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Melatonin madness. Cell 1995, 83, 1059–1062. [Google Scholar] [CrossRef] [Green Version]
- Socaciu, A.I.; Ionuţ, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an ubiquitous metabolic regulator: Functions, mechanisms, and effects on circadian disruption and degenerative diseases. Rev. Endocr. Metab. Disord. 2020, 21, 465–478. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef]
- Bu, S.; Wang, Q.; Sun, J.; Li, X.; Gu, T.; Lai, D. Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. 2020, 11, 644. [Google Scholar] [CrossRef]
- Owino, S.; Buonfiglio, D.; Tchio, C.; Tosini, G. Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Genario, R.; Cipolla-Neto, J.; Bueno, A.A.; Santos, H.O. Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 2021, 163, 105254. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is melatonin the cornucopia of the 21st century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Wade, G.N. Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): Role of pineal gland, melatonin, gonads, and diet. Endocrinology 1984, 114, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Fuentes-Broto, L.; Paredes, S.D.; Reiter, R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: Relation to human obesity. Obes. Rev. 2011, 12, 167–188. [Google Scholar] [CrossRef]
- Tamura, I.; Tamura, H.; Kawamoto-Jozaki, M.; Doi-Tanaka, Y.; Takagi, H.; Shirafuta, Y.; Mihara, Y.; Maekawa, R.; Taketani, T.; Sato, S.; et al. Long-term melatonin treatment attenuates body weight gain with aging in female mice. J. Endocrinol. 2021, 251, 15–25. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Adebisi, E.O.; Adeleye, A.E.; Olofinnade, A.T.; Onaolapo, O.J. Dietary melatonin protects against behavioural, metabolic, oxidative, and organ morphological changes in mice that are fed high-fat, high- sugar diet. Endocr. Metab. Immune. Disord. Drug Targets 2020, 20, 570–583. [Google Scholar] [CrossRef]
- Mendes, C.; Gomes, G.; Belpiede, L.T.; do Carmo Buonfiglio, D.; Motta-Teixeira, L.C.; Amaral, F.G.; Cipolla-Neto, J. The effects of melatonin daily supplementation to aged rats on the ability to withstand cold, thermoregulation and body weight. Life Sci. 2021, 265, 118769. [Google Scholar] [CrossRef]
- Wang, L.; McFadden, J.W.; Yang, G.; Zhu, H.; Lian, H.; Fu, T.; Sun, Y.; Gao, T.; Li, M. Effect of melatonin on visceral fat deposition, lipid metabolism and hepatic lipo-metabolic gene expression in male rats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 787–796. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chiang, P.C.; Chen, Y.L.; Chien, Y.W. Effects of melatonin on lipid metabolism and circulating irisin in Sprague-Dawley rats with diet-induced obesity. Molecules 2020, 25, 3329. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Y.; Luo, X.M.; Ma, Y.; Liu, J.; Wang, H. Intermittent fasting reshapes the gut microbiota and metabolome and reduces weight gain more effectively than melatonin in mice. Front. Nutr. 2021, 8, 784681. [Google Scholar] [CrossRef]
- Xu, P.F.; Wang, J.L.; Hong, F.; Wang, S.; Jin, X.; Xue, T.T.; Jia, L.; Zhai, Y.G. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017, 62, e12399. [Google Scholar] [CrossRef]
- Farias, T.; Paixao, R.I.D.; Cruz, M.M.; de Sa, R.; Simão, J.J.; Antraco, V.J.; Alonso-Vale, M.I.C. Melatonin supplementation attenuates the pro-inflammatory adipokines expression in visceral fat from obese mice induced by a high-fat diet. Cells 2019, 8, 1041. [Google Scholar] [CrossRef] [Green Version]
- Delpino, F.M.; Figueiredo, L.M. Melatonin supplementation and anthropometric indicators of obesity: A systematic review and meta-analysis. Nutrition 2021, 91–92, 111399. [Google Scholar] [CrossRef]
- Mostafavi, S.A.; Solhi, M.; Mohammadi, M.R.; Akhondzadeh, S. Melatonin for reducing weight gain following administration of atypical antipsychotic olanzapine for adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled trial. J. Child Adolesc. Psychopharmacol. 2017, 27, 440–444. [Google Scholar] [CrossRef]
- Romo-Nava, F.; Alvarez-Icaza González, D.; Fresán-Orellana, A.; Saracco Alvarez, R.; Becerra-Palars, C.; Moreno, J.; Ontiveros Uribe, M.P.; Berlanga, C.; Heinze, G.; Buijs, R.M. Melatonin attenuates antipsychotic metabolic effects: An eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar. Disord. 2014, 16, 410–421. [Google Scholar] [CrossRef]
- Modabbernia, A.; Heidari, P.; Soleimani, R.; Sobhani, A.; Roshan, Z.A.; Taslimi, S.; Ashrafi, M.; Modabbernia, M.J. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: Randomized double-blind placebo-controlled study. J. Psychiatr. Res. 2014, 53, 133–140. [Google Scholar] [CrossRef]
- Bahrami, M.; Cheraghpour, M.; Jafarirad, S.; Alavinejad, P.; Asadi, F.; Hekmatdoost, A.; Mohammadi, M.; Yari, Z. The effect of melatonin on treatment of patients with non-alcoholic fatty liver disease: A randomized double blind clinical trial. Complement. Ther. Med. 2020, 52, 102452. [Google Scholar] [CrossRef]
- Treister-Goltzman, Y.; Peleg, R. Melatonin and the health of menopausal women: A systematic review. J. Pineal Res. 2021, 71, e12743. [Google Scholar] [CrossRef]
- Mohammadi, S.; Rastmanesh, R.; Jahangir, F.; Amiri, Z.; Djafarian, K.; Mohsenpour, M.A.; Hassanipour, S.; Ghaffarian-Bahraman, A. Melatonin supplementation and anthropometric indices: A randomized double-blind controlled clinical trial. Biomed. Res. Int. 2021, 2021, 3502325. [Google Scholar] [CrossRef]
- Marqueze, E.C.; Nogueira, L.F.R.; Vetter, C.; Skene, D.J.; Cipolla-Neto, J.; Moreno, C.R.C. Exogenous melatonin decreases circadian misalignment and body weight among early types. J. Pineal Res. 2021, 71, e12750. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Dev, R.; Hui, D.; Palmer, L.; Bruera, E. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: A double-blind placebo-controlled trial. J. Clin. Oncol. 2013, 31, 1271–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafavi, S.; Akhondzadeh, S.; Mohammadi, M.; Keshtkar, A.; Hosseini, S.; Eshraghian, M.; Motlagh, T.; Alipour, R.; Keshavarz, S. Role of melatonin in body weight: A systematic review and meta-analysis. Curr. Pharm. Des. 2017, 23, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Navarro-Alarcón, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vázquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A. Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 2007, 42, 267–271. [Google Scholar] [CrossRef]
- Pita, M.L.; Hoyos, M.; Martin-Lacave, I.; Osuna, C.; Fernández-Santos, J.M.; Guerrero, J.M. Long-term melatonin administration increases polyunsaturated fatty acid percentage in plasma lipids of hypercholesterolemic rats. J. Pineal Res. 2002, 32, 179–186. [Google Scholar] [CrossRef]
- De Farias, T.; Cruz, M.; de Sa, R.; Severi, I.; Perugini, J.; Senzacqua, M.; Cerutti, S.; Giordano, A.; Cinti, S.; Alonso-Vale, M. Melatonin supplementation decreases hypertrophic obesity and inflammation induced by high-fat diet in mice. Front. Endocrinol. 2019, 10, 750. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Lugo, M.J.; Cano, P.; Jiménez-Ortega, V.; Fernández-Mateos, M.; Scacchi, P.; Cardinali, D.; Esquifino, A. Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J. Pineal Res. 2010, 49, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Ou, T.H.; Tung, Y.T.; Yang, T.H.; Chien, Y.W. Melatonin improves fatty liver syndrome by inhibiting the lipogenesis pathway in hamsters with high-fat diet-induced hyperlipidemia. Nutrients 2019, 11, 748. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi-Sartang, M.; Ghorbani, M.; Mazloom, Z. Effects of melatonin supplementation on blood lipid concentrations: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 1943–1954. [Google Scholar] [CrossRef]
- Loloei, S.; Sepidarkish, M.; Heydarian, A.; Tahvilian, N.; Khazdouz, M.; Heshmati, J.; Pouraram, H. The effect of melatonin supplementation on lipid profile and anthropometric indices: A systematic review and meta-analysis of clinical trials. Diabetes Metab. Syndr. 2019, 13, 1901–1910. [Google Scholar] [CrossRef]
- Koziróg, M.; Poliwczak, A.R.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Broncel, M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 2011, 50, 261–266. [Google Scholar] [CrossRef]
- Parandavar, N.; Hojat, M.; Abdali, K.; Keshtgar, S.; Emamghoreishi, M.; Yeganeh, B.S. The effect of melatonin on the lipid levels in menopausal women: A double-blind, controlled, clinical trial. J. Educ. Health Promot. 2018, 7, 144. [Google Scholar] [CrossRef]
- Chan, T.Y.; Tang, P.L. Effect of melatonin on the maintenance of cholesterol homeostasis in the rat. Endocr. Res. 1995, 21, 681–696. [Google Scholar] [CrossRef]
- Müller-Wieland, D.; Behnke, B.; Koopmann, K.; Krone, W. Melatonin inhibits LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. Biochem. Biophys. Res. Commun. 1994, 203, 416–421. [Google Scholar] [CrossRef]
- She, M.H.; Deng, X.J.; Guo, Z.Y.; Laudon, M.; Hu, Z.W.; Liao, D.F.; Hu, X.B.; Luo, Y.; Shen, Q.Y.; Su, Z.H.; et al. NEU-P11, a novel melatonin agonist, inhibits weight gain and improves insulin sensitivity in high-fat/high-sucrose-fed rats. Pharmacol. Res. 2009, 59, 248–253. [Google Scholar] [CrossRef]
- Kanter, M.; Uysal, H.; Karaca, T.; Sagmanligil, H.O. Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch. Toxicol. 2006, 80, 362–369. [Google Scholar] [CrossRef]
- Li, T.; Ni, L.; Zhao, Z.; Liu, X.; Lai, Z.; Di, X.; Xie, Z.; Song, X.; Wang, X.; Zhang, R.; et al. Melatonin attenuates smoking-in duced. hyperglycemia via preserving insulin secretion and hepatic glycogen synthesis in rats. J. Pineal Res. 2018, 64, e12475. [Google Scholar] [CrossRef] [Green Version]
- Lyssenko, V.; Nagorny, C.; Erdos, M.; Wierup, N.; Jonsson, A.; Spégel, P.; Bugliani, M.; Saxena, R.; Fex, M.; Pulizzi, N.; et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 2009, 41, 82–88. [Google Scholar] [CrossRef]
- Shieh, J.; Wu, H.; Cheng, K.; Cheng, J. Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKCzeta-Akt-GSK3beta pathway in hepatic cells. J. Pineal Res. 2009, 47, 339–344. [Google Scholar] [CrossRef]
- Song, J.Y.; Zhang, H.J.; Wang, Z.Y.; Xu, W.L.; Zhong, L.; Cao, J.M.; Yang, J.F.; Tian, Y.; Yu, D.J.; Ji, J.; et al. The role of FABP5 in radiation-induced human skin fibrosis. Radiat. Res. 2018, 189, 177–186. [Google Scholar] [CrossRef]
- Gomes, P.R.L.; Vilas-Boas, E.A.; Leite, E.A.; Munhoz, A.C.; Lucena, C.F.; Amaral, F.G.D.; Carpinelli, A.R.; Cipolla-Neto, J. Melatonin regulates maternal pancreatic remodeling and B-cell function during pregnancy and lactation. J. Pineal Res. 2021, 71, e12717. [Google Scholar] [CrossRef]
- Picinato, M.; Hirata, A.; Cipolla-Neto, J.; Curi, R.; Carvalho, C.; Anhê, G.; Carpinelli, A. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J. Pineal Res. 2008, 44, 88–94. [Google Scholar] [CrossRef]
- Chen, J.; Xia, H.Z.; Zhang, L.; Zhang, H.; Wang, D.; Tao, X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019, 117, 109150. [Google Scholar] [CrossRef]
- Ha, E.; Yim, S.V.; Chung, J.H.; Yoon, K.S.; Kang, I.; Cho, Y.H.; Baik, H. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J. Pineal Res. 2006, 41, 67–72. [Google Scholar] [CrossRef]
- Teodoro, B.; Baraldi, F.; Sampaio, I.; Bomfim, L.; Queiroz, A.; Passos, M.; Carneiro, E.; Alberici, L.; Gomis, R.; Amaral, F.; et al. Melatonin prevents mitochondrial dysfunction and insulin resistance in rat skeletal muscle. J. Pineal Res. 2014, 57, 155–167. [Google Scholar] [CrossRef]
- Bazwinsky-Wutschke, I.; Wolgast, S.; Mühlbauer, E.; Albrecht, E.; Peschke, E. Phosphorylation of cyclic AMP-response element-binding protein (CREB) is influenced by melatonin treatment in pancreatic rat insulinoma β-cells (INS-1). J. Pineal Res. 2012, 53, 344–357. [Google Scholar] [CrossRef]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 2013, 14, 6981–7015. [Google Scholar] [CrossRef] [Green Version]
- Peschke, E.; Bähr, I.; Mühlbauer, E. Experimental and clinical aspects of melatonin and clock genes in diabetes. J. Pineal Res. 2015, 59, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.L.; Wu, H.H.; Liu, N.J.; Cao, X.Y.; Yang, Z.; Lu, B.; Hu, R.M.; Wang, X.C.; Wen, J. Melatonin exerts an inhibitory effect on insulin gene transcription via MTNR1B and the downstream Raf-1/ERK signaling pathway. Int. J. Mol. Med. 2018, 41, 955–961. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.; Andreotti, S.; Farias, T.S.; Torres-Leal, F.; de Proença, A.; Campaña, A.; de Souza, A.; Sertié, R.; Carpinelli, A.; Cipolla-Neto, J.; et al. Metabolic disorders and adipose tissue insulin responsiveness in neonatally STZ-induced diabetic rats are improved by long-term melatonin treatment. Endocrinology 2012, 153, 2178–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Vara, E.; Tresguerres, J. Melatonin can improve insulin resistance and aging-induced pancreas alterations in senescence-accelerated prone male mice (SAMP8). Age 2013, 35, 659–671. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Chen, J.; Gusdon, A.; Song, K.; Li, L.; Qu, S. Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int. J. Endocrinol. 2018, 2018, 2304746. [Google Scholar] [CrossRef] [Green Version]
- Sartori, C.; Dessen, P.; Mathieu, C.; Monney, A.; Bloch, J.; Nicod, P.; Scherrer, U.; Duplain, H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 2009, 150, 5311–5317. [Google Scholar] [CrossRef] [Green Version]
- McMullan, C.; Curhan, G.; Schernhammer, E.; Forman, J. Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol. 2013, 178, 231–238. [Google Scholar] [CrossRef] [Green Version]
- De Luis, D.A.; Izaola, O.; Primo, D.; Aller, R. A circadian rhythm-related MTNR1B genetic variant (rs10830963) modulate body weight change and insulin resistance after 9 months of a high protein/low carbohydrate vs a standard hypocaloric diet. J. Diabetes Complicat. 2020, 34, 107534. [Google Scholar] [CrossRef]
- Liang, Z.X.; Liu, H.K.; Wang, L.S.; Chen, Y.H.; Zhou, T.; Heianza, Y.; Li, W.Q.; Leng, J.H.; Wang, J.; Gao, R.; et al. Maternal MTNR1B genotype, maternal gestational weight gain, and childhood obesity. Am. J. Clin. Nutr. 2020, 111, 360–368. [Google Scholar] [CrossRef]
- Ivanov, D.; Evsyukova, I.; Mazzoccoli, G.; Anderson, G.; Polyakova, V.; Kvetnoy, I.; Carbone, A.; Nasyrov, R. The role of prenatal melatonin in the regulation of childhood obesity. Biology 2020, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.H.; Kim, S.H.; Cho, Y.M.; Go, M.J.; Cho, Y.S.; Choi, S.H.; Moon, M.K.; Jung, H.S.; Shin, H.D.; Kang, H.M.; et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 2012, 61, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Gombert, M.; Codoñer-Franch, P. Melatonin in early nutrition: Long-term effects on cardiovascular system. Int. J. Mol. Sci. 2021, 22, 6809. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J. Pineal Res. 2017, 63, e12431. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; You, W.; Liu, J.; Wang, Y.; Shan, T. Elucidating the regulatory role of melatonin in brown, white, and beige adipocytes. Adv. Nutr. 2020, 11, 447–460. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.; Gallo, C.; de Camargo, L.; de Carvalho, P.; Olesçuck, I.; Macedo, F.; da Cunha, F.; Cipolla-Neto, J.; do Amaral, F. Melatonin multiple effects on brown adipose tissue molecular machinery. J. Pineal Res. 2019, 66, e12549. [Google Scholar] [CrossRef]
- Pan, S.; Guo, Y.; Hong, F.; Xu, P.; Zhai, Y. Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim. Biophys. Acta. Mol. Basis. Dis. 2021, 1868, 166281. [Google Scholar] [CrossRef]
- Yang, W.; Tang, K.; Wang, Y.; Zhang, Y.; Zan, L. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 2017, 7, 15080. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Yu, W.; Wei, W.; Zhang, X.; Tian, Y.; Sherif, M.; Liu, X.; Dong, C.; Wu, W.; Zhang, L.; et al. Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 2019, 60, 767–782. [Google Scholar] [CrossRef]
- Alonso-Vale, M.I.; Peres, S.B.; Vernochet, C.; Farmer, S.R.; Lima, F.B. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBPbeta transcriptional activity. J. Pineal Res. 2009, 47, 221–227. [Google Scholar] [CrossRef]
- González, A.; Alvarez-García, V.; Martínez-Campa, C.; Alonso-González, C.; Cos, S. Melatonin promotes differentiation of 3T3-L1 fibroblasts. J. Pineal Res. 2012, 52, 12–20. [Google Scholar] [CrossRef]
- Zaminy, A.; Kashani, I.R.; Barbarestani, M.; Hedayatpour, A.; Mahmoudi, R.; Vardasbi, S.; Shokrgozar, M.A. Effects of melatonin on the proliferation and differentiation of rat adipose-derived stem cells. Indian J. Plast. Surg. 2008, 41, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zheng, M.; Mo, J.; Li, K.; Yang, X.; Guo, L.; Zhang, X.; Abdalla, B.A.; Nie, Q. Single-cell RNA sequencing of preadipocytes reveals the cell fate heterogeneity induced by melatonin. J. Pineal Res. 2021, 70, e12725. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.; Reiter, R.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Jimenéz-Aranda, A.; Fernández-Vázquez, G.; Mohammad A-Serrano, M.; Reiter, R.; Agil, A. Melatonin improves mitochondrial function in inguinal white adipose tissue of Zücker diabetic fatty rats. J. Pineal Res. 2014, 57, 103–109. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.; Blanca-Herrera, R.; A-Serrano, M.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Agil, A.; Navarro-Alarcon, M.; Ali, F.A.Z.; Albrakati, A.; Salagre, D.; Campoy, C.; Elmahallawy, E.K. Melatonin enhances the mitochondrial functionality of brown adipose tissue in obese-diabetic rats. Antioxidants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Fernández Vázquez, G.; Reiter, R.; Agil, A. Melatonin increases brown adipose tissue mass and function in Zücker diabetic fatty rats: Implications for obesity control. J. Pineal Res. 2018, 64, e12472. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.C.; Mendes, C.; Machado, C.M.L.; Prando, S.; Sapienza, M.T.; Buchpiguel, C.A.; do Amaral, F.G.; Cipolla-Neto, J. Melatonin deficiency decreases brown adipose tissue acute thermogenic capacity of in rats measured by (18)F-FDG PET. Diabetol. Metab. Syndr. 2020, 12, 82. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.; Bueno, C.; Barcelos, I.; de Melo, M.; Lima, M.; Carneiro, C.; Sapienza, M.; Buchpiguel, C.; do Amaral, F.; et al. Melatonin increases brown adipose tissue volume and activity in patients with melatonin deficiency: A proof-of-concept study. Diabetes 2019, 68, 947–952. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.; Tan, T.; Zhu, T.; Wang, J.; Zhang, S.; Zhang, L.; Hu, X.; Liu, G.; Xing, Y. Leptin mediates the effects of melatonin on female reproduction in mammals. J. Pineal Res. 2019, 66, e12559. [Google Scholar] [CrossRef]
- Suriagandhi, V.; Nachiappan, V. Protective effects of melatonin against obesity-induced by leptin resistance. Behav. Brain Res. 2021, 417, 113598. [Google Scholar] [CrossRef]
- Buonfiglio, D.; Tchio, C.; Furigo, I.; Donato, J.; Baba, K.; Cipolla-Neto, J.; Tosini, G. Removing melatonin receptor type 1 signaling leads to selective leptin resistance in the arcuate nucleus. J. Pineal Res. 2019, 67, e12580. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Golic, I.; Korac, A.; Rezzani, R. Melatonin efficacy in obese leptin-deficient mice heart. Nutrients 2017, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Favero, G.; Stacchiotti, A.; Castrezzati, S.; Bonomini, F.; Albanese, M.; Rezzani, R.; Rodella, L.F. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr. Res. 2015, 35, 891–900. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Woźniak, A.; Reiter, R. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: Implications for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef]
- Mansoori, A.; Salimi, Z.; Hosseini, S.A.; Hormoznejad, R.; Jafarirad, S.; Bahrami, M.; Asadi, M. The effect of melatonin supplementation on liver indices in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 52, 102398. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Wang, T.; Wan, X.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin attenuates (-)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. J. Pineal Res. 2015, 59, 497–507. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Chen, J.; Song, K.; Gusdon, A.M.; Li, L.; Bu, L.; Qu, S. Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 2016, 15, 202. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Meng, F.; Francis, H.; Wu, N.; Chen, L.; Kennedy, L.; Zhou, T.; Franchitto, A.; Onori, P.; Gaudio, E.; et al. Melatonin and circadian rhythms in liver diseases: Functional roles and potential therapies. J. Pineal Res. 2020, 68, e12639. [Google Scholar] [CrossRef]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64. [Google Scholar] [CrossRef]
- El Agaty, S.M.; Ibrahim Ahmed, A. Pathophysiological and immunohistochemical analysis of pancreas after renal ischemia/reperfusion injury: Protective role of melatonin. Arch. Physiol. Biochem. 2020, 126, 264–275. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Kot, M.; Jaworek, A.; Nawrot-Porbka, K.; Bonior, J.; Szklarczyk, J. The role of melatonin in pancreatic protection: Could melatonin be used in the treatment of acute pancreatitis? Curr. Pharm. Des. 2014, 20, 4834–4840. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.H.; Lee, D.C. Urine melatonin levels are inversely associated with sarcopenia in postmenopausal women. Menopause 2014, 21, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Oner, J.; Oner, H.; Sahin, Z.; Demir, R.; Ustünel, I. Melatonin is as effective as testosterone in the prevention of soleus muscle atrophy induced by castration in rats. Anat. Rec. 2008, 291, 448–455. [Google Scholar] [CrossRef]
- Salucci, S.; Taurone, S.; Burattini, S.; Gobbi, P.; Clausi, J.; Battistelli, M. Melatonin role in skeletal muscle disorders. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1024–1033. [Google Scholar] [CrossRef]
- Stacchiotti, A.; Favero, G.; Rodella, L.F. Impact of melatonin on skeletal muscle and exercise. Cells 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; You, W.; Shan, T. The regulatory role of melatonin in skeletal muscle. J. Muscle Res. Cell. Motil. 2020, 41, 191–198. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef]
- Tosini, G.; Owino, S.; Guillaume, J.L.; Jockers, R. Understanding melatonin receptor pharmacology: Latest insights from mouse models, and their relevance to human disease. Bioessays 2014, 36, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Ciuculete, D.M.; Schiöth, H.B.; Benedict, C. Associations between chronotype, MTNR1B genotype and risk of type 2 diabetes in UK Biobank. J. Intern. Med. 2020, 287, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ma, J.; Yao, K.; Su, W.; Tan, B.; Wu, X.; Huang, X.; Li, T.; Yin, Y.; Tosini, G.; et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J. Pineal Res. 2020, 69, e12682. [Google Scholar] [CrossRef]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.C.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.P.; Savych, O.; Moroz, Y.S.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef]
- Ferreira, M.A., Jr.; Azevedo, H.; Mascarello, A.; Segretti, N.D.; Russo, E.; Russo, V.; Guimarães, C.R.W. Discovery of ACH-000143: A novel potent and peripherally preferred melatonin receptor agonist that reduces liver triglycerides and steatosis in diet-induced obese rats. J. Med. Chem. 2021, 64, 1904–1929. [Google Scholar] [CrossRef]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef]
- Sun, H.; Huang, F.F.; Qu, S. Melatonin: A potential intervention for hepatic steatosis. Lipids Health Dis. 2015, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Duez, H.; Staels, B. Rev-erb alpha gives a time cue to metabolism. FEBS Lett. 2008, 582, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.; Scott, E. Circadian rhythms, insulin action, and glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Samblas, M.; Milagro, F.; Riezu-Boj, J.; Crujeiras, A.; Martinez, J.; Project, M. Circadian gene methylation profiles are associated with obesity, metabolic disturbances and carbohydrate intake. Chronobiol. Int. 2018, 35, 969–981. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.; Espitia-Bautista, E.; Buijs, R.; Escobar, C. Shift-work: Is time of eating determining metabolic health? Evidence from animal models. Proc. Nutr. Soc. 2018, 77, 199–215. [Google Scholar] [CrossRef]
- McFadden, E.; Jones, M.; Schoemaker, M.; Ashworth, A.; Swerdlow, A. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am. J. Epidemiol. 2014, 180, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Uchida, D.; Itoh, N. Low-carbohydrate, high-protein diet affects rhythmic expression of gluconeogenic regulatory and circadian clock genes in mouse peripheral tissues. Chronobiol. Int. 2012, 29, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinogradova, I.; Anisimov, V. Melatonin prevents the development of the metabolic syndrome in male rats exposed to different light/dark regimens. Biogerontology 2013, 14, 401–409. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Chen, Q.; Liu, S.; Xu, W.; Shang, W.; Wang, L.; Yu, J. Melatonin alleviates glucose and lipid metabolism disorders in Guinea pigs caused by different artificial light rhythms. J. Diabetes Res. 2020, 2020, 4927403. [Google Scholar] [CrossRef]
- Choi, Y.; Nakamura, Y.; Akazawa, N.; Park, I.; Kwak, H.B.; Tokuyama, K.; Maeda, S. Effects of nocturnal light exposure on circadian rhythm and energy metabolism in healthy adults: A randomized crossover trial. Chronobiol. Int. 2021, 1–11. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Xu, P.; Xue, T.; Wang, J.; Guo, Y.; Jia, L.; Qiao, X.; Li, L.; Zhai, Y. Melatonin orchestrates lipid homeostasis through the hepatointestinal circadian clock and microbiota during constant light exposure. Cells 2020, 9, 489. [Google Scholar] [CrossRef] [Green Version]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Luo, D.; Sun, C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J. Pineal Res. 2017, 62, e12383. [Google Scholar] [CrossRef]
- Shi, L.; Li, N.; Bo, L.; Xu, Z. Melatonin and hypothalamic-pituitary-gonadal axis. Curr. Med. Chem. 2013, 20, 2017–2031. [Google Scholar] [CrossRef]
- Oladele, C.A.; Akintayo, C.O.; Badejogbin, O.C.; Oniyide, A.A.; Omoaghe, A.O.; Agunbiade, T.B.; Olaniyi, K.S. Melatonin ameliorates endocrine dysfunction and defective sperm integrity associated with high-fat diet-induced obesity in male Wistar rats. Andrologia 2021, e14242. [Google Scholar] [CrossRef]
- Mills, J.; Kuohung, W. Impact of circadian rhythms on female reproduction and infertility treatment success. Curr Opin Endocrinol. Diabetes Obes. 2019, 26, 317–321. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Ren, Y.; Li, C. High ambient temperature disrupted the circadian rhythm of reproductive hormones and changed the testicular expression of steroidogenesis genes and clock genes in male mice. Mol. Cell. Endocrinol. 2020, 500, 110639. [Google Scholar] [CrossRef]
- Sciarra, F.; Franceschini, E.; Campolo, F.; Gianfrilli, D.; Pallotti, F.; Paoli, D.; Isidori, A.M.; Venneri, M.A. Disruption of circadian rhythms: A crucial factor in the etiology of infertility. Int. J. Mol. Sci. 2020, 21, 3943. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z.; Wang, X.; Liu, Q.; Gu, L.; Oka, Y.; Lin, C. Beyond the photocycle-how cryptochromes regulate photoresponses in plants? Curr. Opin. Plant. Biol. 2018, 45, 120–126. [Google Scholar] [CrossRef] [Green Version]
- Wahl, S.; Engelhardt, M.; Schaupp, P.; Lappe, C.; Ivanov, I.V. The inner clock-Blue light sets the human rhythm. J. Biophotonics 2019, 12, e201900102. [Google Scholar] [CrossRef]
- Yoshiuchi, I. Analysis of evolution and ethnic diversity at glucose-associated SNPs of circadian clock-related loci with cryptochrome 1, cryptochrome 2, and melatonin receptor 1B. Biochem. Genet. 2021, 59, 1173–1184. [Google Scholar] [CrossRef]
- Jastroch, M.; Ussar, S.; Keipert, S. Gut microbes controlling blood sugar: No fire required! Cell Metab. 2020, 31, 443–444. [Google Scholar] [CrossRef]
- Barrenetxe, J.; Delagrange, P.; Martínez, J. Physiological and metabolic functions of melatonin. J. Physiol. Biochem. 2004, 60, 61–72. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Ren, W.; Wang, P.; Yan, J.; Liu, G.; Zeng, B.; Hussain, T.; Peng, C.; Yin, J.; Li, T.; Wei, H.; et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J. Pineal Res. 2018, 64, e12448. [Google Scholar] [CrossRef]
- Yildirim, A.; Arabacı Tamer, S.; Sahin, D.; Bagriacik, F.; Kahraman, M.M.; Onur, N.D.; Cayirli, Y.B.; Kaya, Ö.T.C.; Aksu, B.; Akdeniz, E.; et al. The effects of antibiotics and melatonin on hepato-intestinal inflammation and gut microbial dysbiosis induced by a short-term high-fat diet consumption in rats. Br. J. Nutr. 2019, 122, 841–855. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [Green Version]
- Rong, B.; Wu, Q.; Reiter, R.J.; Sun, C. The mechanism of oral melatonin ameliorates intestinal and adipose lipid dysmetabolism through reducing Escherichia Coli-derived lipopolysaccharide. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1643–1667. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. Msystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Bacaro, V.; Ballesio, A.; Cerolini, S.; Vacca, M.; Poggiogalle, E.; Donini, L.M.; Lucidi, F.; Lombardo, C. Sleep duration and obesity in adulthood: An updated systematic review and meta-analysis. Obes. Res. Clin. Pract. 2020, 14, 301–309. [Google Scholar] [CrossRef]
- Shih, D.P.; Lin, P.Y.; Liang, W.M.; Tseng, P.C.; Kuo, H.W.; Wang, J.Y. Sleep duration and effort-reward imbalance (ERI) associated with obesity and type II diabetes mellitus (T2DM) among Taiwanese middle-aged public servants. Int. J. Environ. Res. Public Health 2020, 17, 6577. [Google Scholar] [CrossRef]
- Lao, X.Q.; Liu, X.; Deng, H.B.; Chan, T.C.; Ho, K.F.; Wang, F.; Vermeulen, R.; Tam, T.; Wong, M.C.S.; Tse, L.A.; et al. Sleep quality, sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60,586 adults. J. Clin. Sleep Med. 2018, 14, 109–117. [Google Scholar] [CrossRef] [Green Version]
- St-Onge, M.P. Sleep-obesity relation: Underlying mechanisms and consequences for treatment. Obes. Rev. 2017, 18 (Suppl. S1), 34–39. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huysmans, S.; De Hert, M.; Desplenter, F. Melatonin and sleep disorders: Overview of literature and testing in psychiatric practice. Tijdschrift voor Psychiatrie 2019, 61, 854–861. [Google Scholar] [PubMed]
- Riha, R.L. The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr. Opin. Pulm. Med. 2018, 24, 543–548. [Google Scholar] [CrossRef]
- Liu, C.; Weaver, D.R.; Jin, X.; Shearman, L.P.; Pieschl, R.L.; Gribkoff, V.K.; Reppert, S.M. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 1997, 19, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Sharma, R.; Sahota, P.; Thakkar, M.M. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. J. Pineal Res. 2018, 65, e12498. [Google Scholar] [CrossRef]
- Gombert, M.; Martin-Carbonell, V.; Pin-Arboledas, G.; Carrasco-Luna, J.; Carrasco-García, Á.; Codoñer-Franch, P. Melatonin levels in children with obesity are associated with metabolic risk and inflammatory parameters. Nutrients 2021, 13, 3629. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919, quiz 920. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [Green Version]
- Simões, D.; Riva, P.; Peliciari-Garcia, R.; Cruzat, V.; Graciano, M.; Munhoz, A.; Taneda, M.; Cipolla-Neto, J.; Carpinelli, A. Melatonin modifies basal and stimulated insulin secretion via NADPH oxidase. J. Endocrinol. 2016, 231, 235–244. [Google Scholar] [CrossRef]
- Mesri Alamdari, N.; Mahdavi, R.; Roshanravan, N.; Lotfi Yaghin, N.; Ostadrahimi, A.R.; Faramarzi, E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm. Metab. Res. 2015, 47, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Gonzaga, N.A.; Awata, W.M.C.; Ficher, S.P.; Assis, V.O.; Alves, J.V.; Tostes, R.C.; Tirapelli, C.R. Melatonin reverses the loss of the anticontractile effect of perivascular adipose tissue in obese rats. J. Pineal Res. 2021, 70, e12710. [Google Scholar] [CrossRef]
- Shah, S.A.; Khan, M.; Jo, M.H.; Jo, M.G.; Amin, F.U.; Kim, M.O. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci. Ther. 2017, 23, 33–44. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef]
- Promsan, S.; Lungkaphin, A. The roles of melatonin on kidney injury in obese and diabetic conditions. Biofactors 2020, 46, 531–549. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Terron, M.P.; Flores, L.J.; Czarnocki, Z. Melatonin and its metabolites: New findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 2007, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hermoso, D.; Shimada, L.; Gilglioni, E.; Constantin, J.; Mito, M.; Hermoso, A.; Salgueiro-Pagadigorria, C.; Iwamoto, E. Melatonin protects female rats against steatosis and liver oxidative stress induced by oestrogen deficiency. Life Sci. 2016, 157, 178–186. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Maiocchi, S.L.; Morris, J.C.; Rees, M.D.; Thomas, S.R. Regulation of the nitric oxide oxidase activity of myeloperoxidase by pharmacological agents. Biochem. Pharmacol. 2017, 135, 90–115. [Google Scholar] [CrossRef]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Tooze, S.; Dikic, I. Autophagy captures the Nobel Prize. Cell 2016, 167, 1433–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Ledo, A.; Luxán-Delgado, B.; Caballero, B.; Potes, Y.; Rodríguez-González, S.; Boga, J.A.; Coto-Montes, A.; García-Macia, M. Melatonin ameliorates autophagy impairment in a metabolic syndrome model. Antioxidants 2021, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Vega, N.; Marín-Aragón, C.; López-Aguilar, I.; Ibarra-Reynoso, L.; Pérez-Luque, E.; Malacara, J. Analysis of the percentages of monocyte subsets and ILC2s, their relationships with metabolic variables and response to hypocaloric restriction in obesity. PLoS ONE 2020, 15, e0228637. [Google Scholar] [CrossRef] [Green Version]
- Bonomini, F.; Dos Santos, M.; Veronese, F.; Rezzani, R. NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-Induced lupus nephritis. Int. J. Mol. Sci. 2019, 20, 3466. [Google Scholar] [CrossRef] [Green Version]
- Cano Barquilla, P.; Pagano, E.S.; Jiménez-Ortega, V.; Fernández-Mateos, P.; Esquifino, A.I.; Cardinali, D.P. Melatonin normalizes clinical and biochemical parameters of mild inflammation in diet-induced metabolic syndrome in rats. J. Pineal Res. 2014, 57, 280–290. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef]
- Yawoot, N.; Govitrapong, P.; Tocharus, C.; Tocharus, J. Ischemic stroke, obesity, and the anti-inflammatory role of melatonin. Biofactors 2021, 47, 41–58. [Google Scholar] [CrossRef]
- Mauriz, J.; Collado, P.; Veneroso, C.; Reiter, R.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation-story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gan, L.; Xu, Y.; Luo, D.; Ren, Q.; Wu, S.; Sun, C. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J. Pineal Res. 2017, 63, e12414. [Google Scholar] [CrossRef]
- Pivonello, C.; Negri, M.; Patalano, R.; Amatrudo, F.; Montò, T.; Liccardi, A.; Graziadio, C.; Muscogiuri, G.; Pivonello, R.; Colao, A. The role of melatonin in the molecular mechanisms underlying metaflammation and infections in obesity: A narrative review. Obes. Rev. 2021, e13390. [Google Scholar] [CrossRef]
- Mendes, K.; Lelis, D.; Santos, S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Liu, L.; Zhao, Y.; Yang, L.; Cheng, J.; Hua, R.; Zhang, Z.; Li, Q. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J. Pineal Res. 2020, 69, e12690. [Google Scholar] [CrossRef]
- Favero, G.; Franco, C.; Stacchiotti, A.; Rodella, L.F.; Rezzani, R. Sirtuin1 role in the melatonin protective effects against obesity-related heart injury. Front. Physiol. 2020, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- Ireland, K.E.; Maloyan, A.; Myatt, L. Melatonin improves mitochondrial respiration in syncytiotrophoblasts from placentas of obese women. Reprod. Sci. 2018, 25, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Prado, N.; Ferder, L.; Manucha, W.; Diez, E. Anti-inflammatory effects of melatonin in obesity and hypertension. Curr. Hypertens. Rep. 2018, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Rodriguez, M.; Reiter, R.J. Multiple sclerosis: Melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int. J. Mol. Sci. 2019, 20, 5500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacchiotti, A.; Favero, G.; Giugno, L.; Lavazza, A.; Reiter, R.J.; Rodella, L.F.; Rezzani, R. Mitochondrial and metabolic dysfunction in renal convoluted tubules of obese mice: Protective role of melatonin. PLoS ONE 2014, 9, e111141. [Google Scholar] [CrossRef] [Green Version]
- El-Missiry, M.A.; El-Missiry, Z.M.A.; Othman, A.I. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of COVID-19. Eur. J. Pharmacol. 2020, 882, 173329. [Google Scholar] [CrossRef]
- McFadden, K.L.; Cornier, M.A.; Tregellas, J.R. The role of alpha-7 nicotinic receptors in food intake behaviors. Front. Psychol. 2014, 5, 553. [Google Scholar] [CrossRef] [Green Version]
- Nogueiras, R.; Romero-Picó, A.; Vazquez, M.J.; Novelle, M.G.; López, M.; Diéguez, C. The opioid system and food intake: Homeostatic and hedonic mechanisms. Obes. Facts 2012, 5, 196–207. [Google Scholar] [CrossRef]
- Han, J.; Xu, Y.; Yu, C.X.; Shen, J.; Wei, Y.M. Melatonin reverses the expression of morphine-induced conditioned place preference through its receptors within central nervous system in mice. Eur. J. Pharmacol. 2008, 594, 125–131. [Google Scholar] [CrossRef]
- Peciña, M.; Karp, J.F.; Mathew, S.; Todtenkopf, M.S.; Ehrich, E.W.; Zubieta, J.K. Endogenous opioid system dysregulation in depression: Implications for new therapeutic approaches. Mol. Psychiatry 2019, 24, 576–587. [Google Scholar] [CrossRef]
- Novais, A.A.; Chuffa, L.G.A.; Zuccari, D.; Reiter, R.J. Exosomes and melatonin: Where their destinies intersect. Front. Immunol. 2021, 12, 692022. [Google Scholar] [CrossRef]
- Heo, J.S.; Lim, J.Y.; Yoon, D.W.; Pyo, S.; Kim, J. Exosome and melatonin additively attenuates inflammation by transferring miR-34a, miR-124, and miR-135b. Biomed. Res. Int. 2020, 2020, 1621394. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The safety of melatonin in humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Andersen, I.M.; Kaczmarska, J.; McGrew, S.G.; Malow, B.A. Melatonin for insomnia in children with autism spectrum disorders. J. Child. Neurol. 2008, 23, 482–485. [Google Scholar] [CrossRef]
- Rzepka-Migut, B.; Paprocka, J. Efficacy and safety of melatonin treatment in children with autism spectrum disorder and attention-deficit/hyperactivity disorder-a review of the literature. Brain Sci. 2020, 10, 219. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.; Smits, M.G.; Tang, X.; Kuang, L.; Meng, H.; Ni, S.; Xiao, M.; Zhou, X. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: A meta-analysis of randomized controlled trials. Sleep Med. 2020, 68, 1–8. [Google Scholar] [CrossRef]
- Malow, B.A.; Findling, R.L.; Schroder, C.M.; Maras, A.; Breddy, J.; Nir, T.; Zisapel, N.; Gringras, P. Sleep, growth, and puberty after 2 years of prolonged-release melatonin in children with autism spectrum disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2021, 60, 252–261.e3. [Google Scholar] [CrossRef] [Green Version]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and safety of intravenous, intravesical, rectal, transdermal, and vaginal melatonin in healthy female volunteers: A cross-over study. Pharmacology 2021, 106, 169–176. [Google Scholar] [CrossRef]
| Subjects | Diet | Melatonin Administration | Body Weight | Other Related Effects | References |
|---|---|---|---|---|---|
| Female ICR mice | Normal | 100 μg/mL/day in drinking water for 43 weeks | Decrease | Decrease abdominal fat deposition | Tamura et al. [15] |
| ICR mice | High-fat/ high-sugar | 2.5–10 mg/kg/day in diet for 7 weeks | Decrease | Decrease levels of glucose, insulin, leptin | Onaolapo et al. [16] |
| Aged Wistar rats | Normal | 10 mg/kg/day in drinking water for 16 weeks | Minor increase | Increase the thermogenic ability | Mendes et al. [17] |
| Sprague- Dawley rats | Normal | 10 mg/kg/day by gavage for 12 weeks | Decrease | Decrease adipose deposition | Wang et al. [18] |
| Sprague- Dawley rats | High-fat | 10 or 50 mg/kg/day in drinking water for 8 weeks | Decrease | Reduced serum TG | Tung et al. [19] |
| C57BL/6J mice | Normal | 10 mg/kg/day in drinking water for 15 weeks | No effects | Improve intestinal Allobaclum content | Liu et al. [20] |
| C57BL/6J mice | High-fat | 50 mg/kg/day by gavage for 10 weeks | Decrease | Decrease WAT weight | Xu et al. [21] |
| C57BL/6 mice | High-fat | 1 mg/kg/day in water for 10 weeks | Decrease | Decrease fat deposition and adipocytes size | Farias et al. [22] |
| Subjects | Therapeutic Drug and Its Effect on Weight | Melatonin Administration | Comparison | Improving Effect of Melatonin | References |
|---|---|---|---|---|---|
| Adolescent patients with bipolar disorder | Olanzapine and lithium carbonate (weight gain) | 3 mg/day for 6 or 12 weeks | Placebo | Reduce weight gain | Mostafavi et al. [24] |
| Patients with bipolar disorder or schizophrenia | SGAs (weight gain) | 5 mg/day for 8 weeks | Placebo | Reduce weight gain | Romo-Nava et al. [25] |
| Adult patients with schizophrenia | Olanzapine (weight gain) | 3 mg/day for 8 weeks | Placebo | Reduce weight gain | Modabbernia et al. [26] |
| 55 patients with NAFLD | / | 6 mg/day (1 h before sleep) for 12 weeks | Placebo | Reduce body weight | Bahrami et al. [27] |
| Menopausal women | / | 5–8 mg/day for 6–12 months | Placebo | Reduce BMI | Treister-Goltzman et al. [28] |
| 38 adults with overweight or obesity | / | 3 mg/day for 12 weeks | Placebo | Reduce body weight | Mohammadi et al. [29] |
| Overweight women on night shift | / | 3 mg/day while sleeping for 12 weeks | Placebo | Reduce body weight | Marqueze et al. [30] |
| Patients with cancer and cachexia | / | 20 mg/day for 28 days | Placebo | No effects | Del Fabbro et al. [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Q.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 2022, 23, 218. https://doi.org/10.3390/ijms23010218
Guan Q, Wang Z, Cao J, Dong Y, Chen Y. Mechanisms of Melatonin in Obesity: A Review. International Journal of Molecular Sciences. 2022; 23(1):218. https://doi.org/10.3390/ijms23010218
Chicago/Turabian StyleGuan, Qingyun, Zixu Wang, Jing Cao, Yulan Dong, and Yaoxing Chen. 2022. "Mechanisms of Melatonin in Obesity: A Review" International Journal of Molecular Sciences 23, no. 1: 218. https://doi.org/10.3390/ijms23010218
APA StyleGuan, Q., Wang, Z., Cao, J., Dong, Y., & Chen, Y. (2022). Mechanisms of Melatonin in Obesity: A Review. International Journal of Molecular Sciences, 23(1), 218. https://doi.org/10.3390/ijms23010218







