Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review
Abstract
:1. Introduction
2. Principles of Metasurfaces Biosensing
3. Tumor Marker Screening Based on Plasmonic Metasurfaces
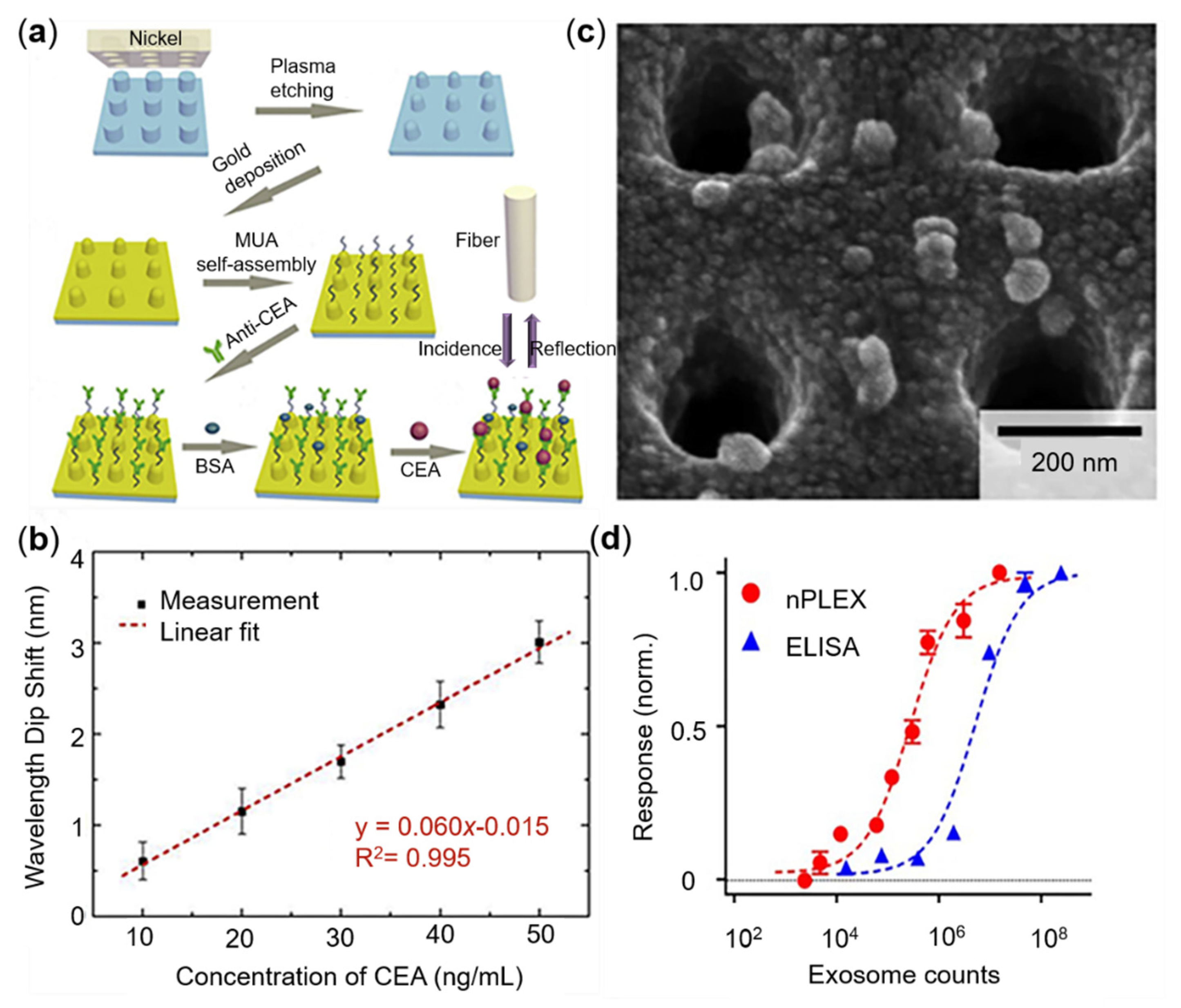
3.1. CEA Sensing
3.2. PSA Sensing
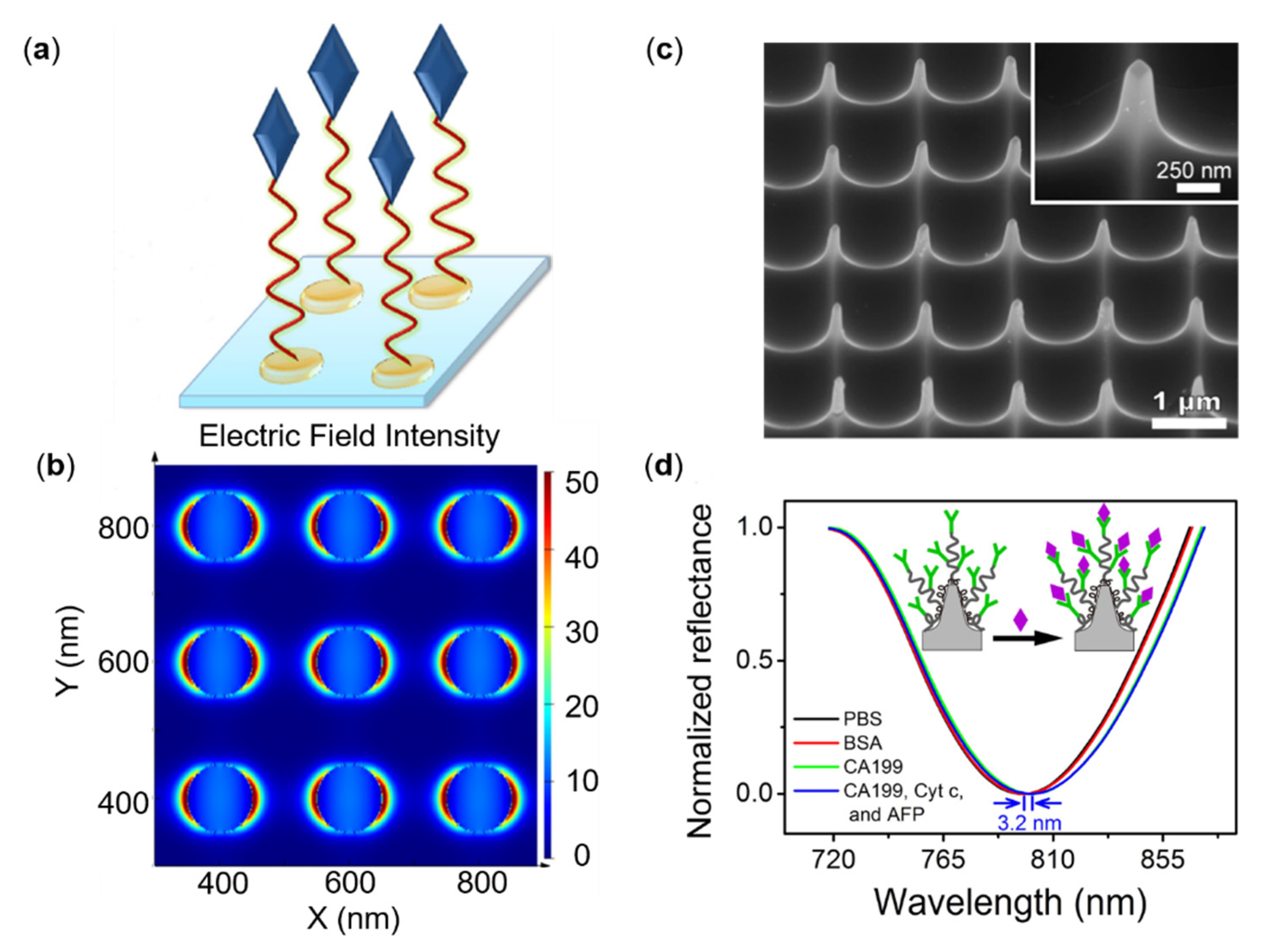
3.3. Other Protein Tumor Markers Sensing
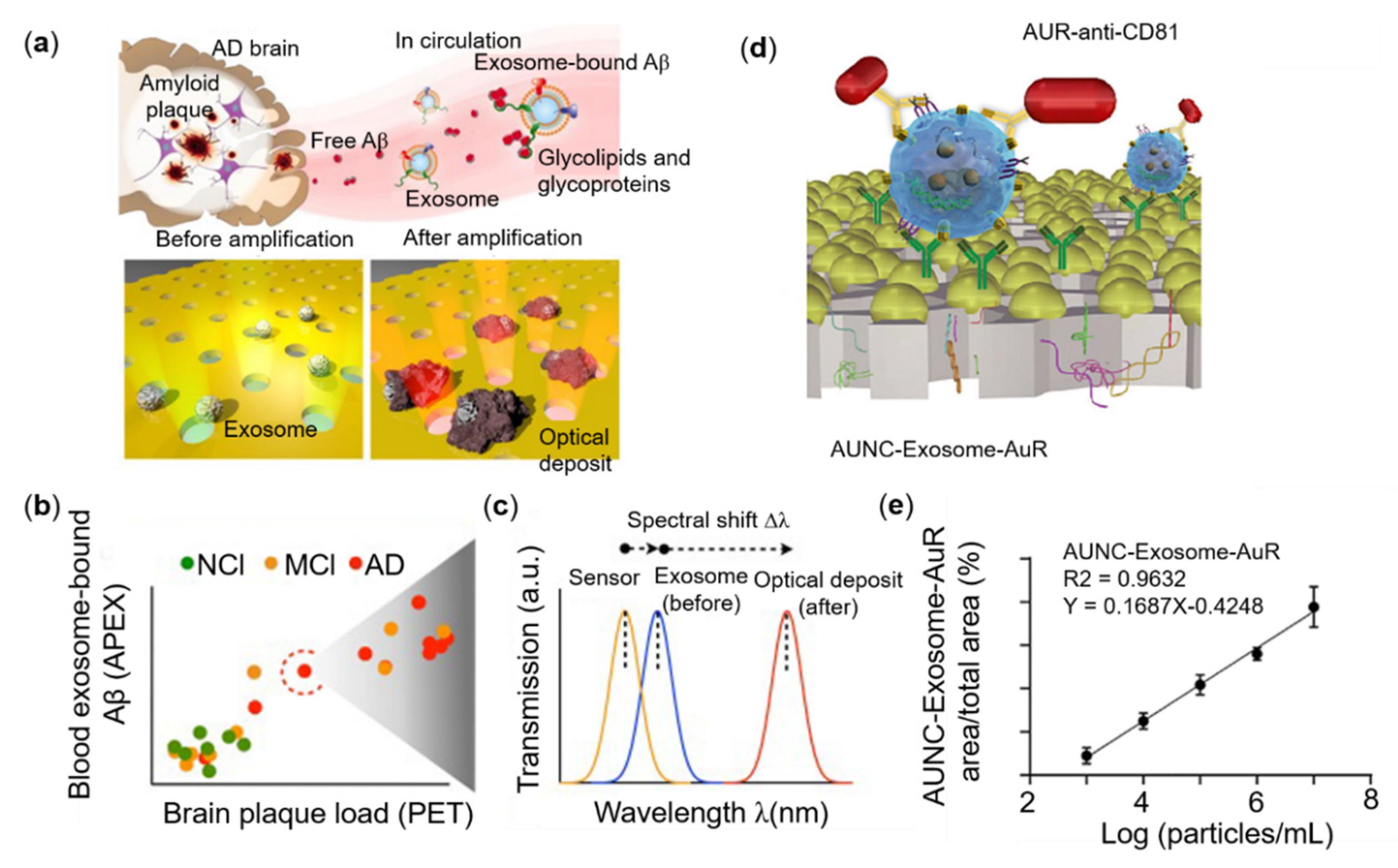
3.4. Tumor-Derived Exosome Sensing
4. COVID-19 Sensing Based on Plasmonic Metastructures
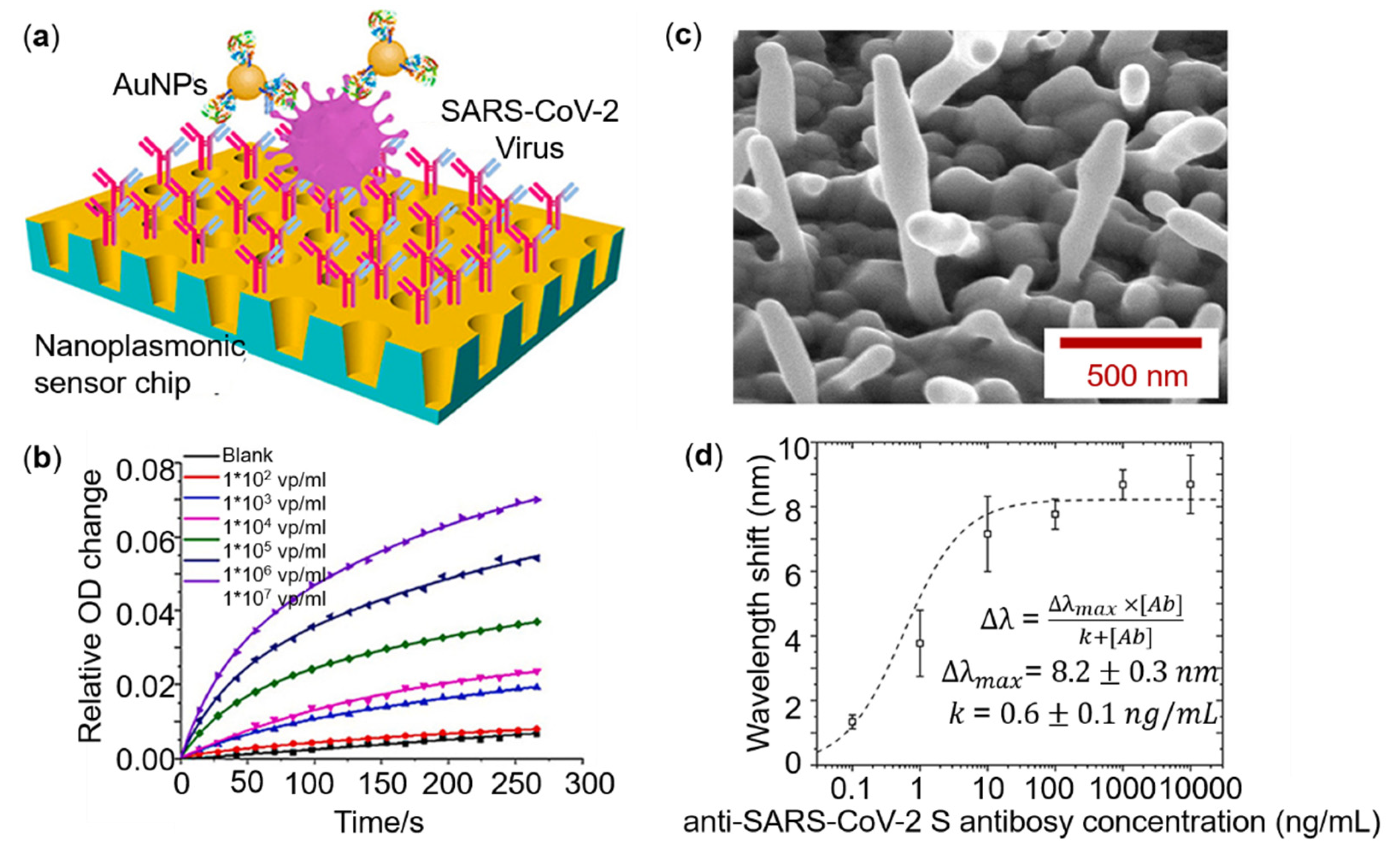
4.1. Detection Based on Metasurfaces
4.2. Detection Based on Self-Assembled Metastructures
5. Conclusions and Future Trend
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, R.W. XLII. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Philos. Mag. Ser. 1902, 4, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Fano, U. The theory of anomalous diffraction gratings and of quasi-stationary waves on metallic surfaces (Sommerfeld’s waves). J. Opt. Soc. Am. 1941, 31, 213–222. [Google Scholar] [CrossRef]
- Ritchie, R.H. Plasma losses by fast electrons in thin films. Phys. Rev. 1957, 106, 874. [Google Scholar] [CrossRef]
- Hessel, A.; Oliner, A. A new theory of Wood’s anomalies on optical gratings. Appl. Opt. 1965, 4, 1275–1297. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Maier, S.A.; Brongersma, M.L.; Kik, P.G.; Meltzer, S.; Requicha, A.A.; Atwater, H.A. Plasmonics—A route to nanoscale optical devices. Adv. Mater. 2001, 13, 1501–1505. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Phys. A Hadron. Nucl. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative decay of non-radiative surface plasmons excited by light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, L.; Lu, M.; Yuan, H.; Long, Z.; Peng, W.; Xu, T. Comparative investigation of sensing behaviors between gap andlattice plasmon modes in a metallic nanoring array. Nanoscale 2018, 10, 548–555. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Genet, C.; Bozhevolnyi, S.I. Surface-plasmon circuitry. Phys. Today 2008, 61, 44. [Google Scholar] [CrossRef] [Green Version]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Gramotnev, D.K.; Bozhevolnyi, S.I. Plasmonics beyond the diffraction limit. Nat. Photonics 2010, 4, 83–91. [Google Scholar] [CrossRef]
- Mauriz, E.; Dey, P.; Lechuga, L.M. Advances in nanoplasmonic biosensors for clinical applications. Analyst 2019, 144, 7105–7129. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, G.; Hayat, A.; Andreescu, S. Portable nanoparticle-based sensors for food safety assessment. Sensors 2015, 15, 30736–30758. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Esfandyarpour, R.; Esfandyarpour, H.; Harris, J.S.; Davis, R.W. Simulation and fabrication of a new novel 3D injectable biosensor for high throughput genomics and proteomics in a lab-on-a-chip device. Nanotechnology 2013, 24, 465301. [Google Scholar] [CrossRef] [Green Version]
- Qasim, M.; Lim, D.-J.; Park, H.; Na, D. Nanotechnology for diagnosis and treatment of infectious diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef] [PubMed]
- Cella, L.N.; Chen, W.; Myung, N.V.; Mulchandani, A. Single-walled carbon nanotube-based chemiresistive affinity biosensors for small molecules: Ultrasensitive glucose detection. J. Am. Chem. Soc. 2010, 132, 5024–5026. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.; Wang, X.; Zhao, Q.Q.; Zhu, J.F.; Li, C.W.; He, Y.H.; Hu, S.; Sartin, M.M.; Yan, S.; Ren, B. Probing nanoscale spatial distribution of plasmonically excited hot carriers. Nat. Commun. 2020, 11, 4211. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, A.B.; Jonsson, M.P. Performance of Nanoplasmonic Biosensors. In Nanoplasmonic Sensors; Dmitriev, A., Ed.; Springer: New York, NY, USA, 2012; pp. 231–265. [Google Scholar]
- Consales, M.; Quero, G.; Spaziani, S.; Principe, M.; Micco, A.; Galdi, V.; Cutolo, A.; Cusano, A. Metasurface-Enhanced Lab-on-Fiber Biosensors. Laser Photonics Rev. 2020, 14, 2000180. [Google Scholar] [CrossRef]
- Motogaito, A.; Mito, S.; Miyake, H.; Hiramatsu, K. Detecting high-refractive-index media using surface plasmon sensor with one-dimensional metal diffraction grating. Opt. Photonics J. 2016, 6, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Špačková, B.; Wrobel, P.; Bocková, M.; Homola, J. Optical biosensors based on plasmonic nanostructures: A review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Gao, M.; Yang, W.; Wang, Z.; Lin, S.; Zhu, J.; Yang, Z. Plasmonic resonance-linewidth shrinkage to boost biosensing. Photonics Res. 2020, 8, 1226–1235. [Google Scholar] [CrossRef]
- Udupi, A.; Madhava, S.K. Plasmonic Coupler and Multiplexer/Demultiplexer Based on Nano-Groove-Arrays. Plasmonics 2021, 16, 1685–1692. [Google Scholar] [CrossRef]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A.; Kaźmierczak, A.; Piramidowicz, R. A Numerical Investigation of a Plasmonic Sensor Based on a Metal-Insulator-Metal Waveguide for Simultaneous Detection of Biological Analytes and Ambient Temperature. Nanomaterials 2021, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Chou Chau, Y.-F.; Ming, T.Y.; Chou Chao, C.-T.; Thotagamuge, R.; Kooh, M.R.R.; Huang, H.J.; Lim, C.M.; Chiang, H.-P. Significantly enhanced coupling effect and gap plasmon resonance in a MIM-cavity based sensing structure. Sci. Rep. 2021, 11, 18515. [Google Scholar] [CrossRef]
- Butt, M.A.; Kaźmierczak, A.; Kazanskiy, N.L.; Khonina, S.N. Metal-Insulator-Metal Waveguide-Based Racetrack Integrated Circular Cavity for Refractive Index Sensing Application. Electronics 2021, 10, 1419. [Google Scholar] [CrossRef]
- Ozbay, E. Plasmonics: Merging photonics and electronics at nanoscale dimensions. Science 2006, 311, 189–193. [Google Scholar] [CrossRef]
- Song, E.Y.; Lee, G.Y.; Park, H.; Lee, K.; Kim, J.; Hong, J.; Kim, H.; Lee, B. Compact generation of airy beams with C-aperture metasurface. Adv. Opt. Mater. 2017, 5, 1601028. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Hesselink, L. Solenoidal optical forces from a plasmonic Archimedean spiral. Phys. Rev. A 2019, 100, 013857. [Google Scholar] [CrossRef]
- Hong, Y.; Huh, Y.-M.; Yoon, D.S.; Yang, J. Nanobiosensors based on localized surface plasmon resonance for biomarker detection. J. Nanomater. 2012, 2012, 759830. [Google Scholar] [CrossRef] [Green Version]
- Traverso, A.J.; Huang, J.; Peyronel, T.; Yang, G.; Tiecke, T.G.; Mikkelsen, M.H. Low-loss, centimeter-scale plasmonic metasurface for ultrafast optoelectronics. Optica 2021, 8, 202–207. [Google Scholar] [CrossRef]
- Luo, S.; Li, Q.; Yang, Y.; Chen, X.; Wang, W.; Qu, Y.; Qiu, M. Controlling fluorescence emission with split-ring-resonator-based plasmonic metasurfaces. Laser Photonics Rev. 2017, 11, 1600299. [Google Scholar] [CrossRef]
- Fore, S.; Yuen, Y.; Hesselink, L.; Huser, T. Pulsed-interleaved excitation FRET measurements on single duplex DNA molecules inside C-shaped nanoapertures. Nano Lett. 2007, 7, 1749–1756. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Miroshnichenko, A.E.; Brongersma, M.L.; Kivshar, Y.S.; Luk’yanchuk, B. Optically Resonant Dielectric Nanostructures. Science 2016, 354, 6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanaga, M. All-Dielectric Metasurface Fluorescence Biosensors for High-Sensitivity Antibody/Antigen Detection. ACS Nano 2020, 14, 17458–17467. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. 2008, 26, 4012. [Google Scholar] [CrossRef]
- Lambert, R. Endoscopy in screening for digestive cancer. World J. Gatrointest Endcosc. 2012, 4, 518. [Google Scholar] [CrossRef]
- Horigome, H.; Nomura, T.; Saso, K.; Itoh, M.; Joh, T.; Ohara, H. Limitations of imaging diagnosis for small hepatocellular carcinoma: Comparison with histological findings. J. Gastroenterol. Hepatol. 1999, 14, 559–565. [Google Scholar] [CrossRef]
- Raiko, I.; Sander, I.; Weber, D.G.; Raulf-Heimsoth, M.; Gillissen, A.; Kollmeier, J.; Scherpereel, A.; Brüning, T.; Johnen, G. Development of an enzyme-linked immunosorbent assay for the detection of human calretinin in plasma and serum of mesothelioma patients. BMC Cancer 2010, 10, 242. [Google Scholar] [CrossRef]
- Lind, K.; Kubista, M. Development and evaluation of three real-time immuno-PCR assemblages for quantification of PSA. J. Immunol. Methods. 2005, 304, 107–116. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, L.; Zhao, Y.; Wang, Y.; Peng, Y.; Zhao, S. Multiplexed detection of tumor markers with multicolor quantum dots based on fluorescence polarization immunoassay. Talanta 2012, 92, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.-L.; Grenier, J.; Daurès, J.-P.; Daver, A.; Pujol, H.; Michel, F.-B. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993, 53, 61–66. [Google Scholar] [PubMed]
- Aisner, D.L.; Sams, S.B. The role of cytology specimens in molecular testing of solid tumors: Techniques, limitations, and opportunities. Diagn. Cytopathol. 2012, 40, 511–524. [Google Scholar] [CrossRef]
- Greillier, L.; Baas, P.; Welch, J.J.; Hasan, B.; Passioukov, A. Biomarkers for malignant pleural mesothelioma. Mol. Diagn. Ther. 2008, 12, 375–390. [Google Scholar] [CrossRef]
- Scherpereel, A.; Lee, Y.G. Biomarkers for mesothelioma. Curr. Opin. Pulm. Med. 2007, 13, 339–343. [Google Scholar] [CrossRef]
- Park, E.-K.; Sandrini, A.; Yates, D.H.; Creaney, J.; Robinson, B.W.; Thomas, P.S.; Johnson, A.R. Soluble mesothelin-related protein in an asbestos-exposed population: The dust diseases board cohort study. Am. J. Resp. Crit. Care 2008, 178, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Hagiwara, Y.; Sonoue, K.; Segawa, T.; Miyashita, K.; Maeda, M.; Izumi, H.; Masuda, K.; Hirabayashi, M.; Moroboshi, T. Sensitive and specific new enzyme-linked immunosorbent assay for N-ERC/mesothelin increases its potential as a useful serum tumor marker for mesothelioma. Clin. Cancer Res. 2008, 14, 1431–1437. [Google Scholar] [CrossRef] [Green Version]
- Porcel, J.M.; Vives, M.; Esquerda, A.; Salud, A.; Pérez, B.; Rodríguez-Panadero, F. Use of a panel of tumor markers (carcinoembryonic antigen, cancer antigen 125, carbohydrate antigen 15–3, and cytokeratin 19 fragments) in pleural fluid for the differential diagnosis of benign and malignant effusions. Chest 2004, 126, 1757–1763. [Google Scholar] [CrossRef]
- King, J.; Thatcher, N.; Pickering, C.; Hasleton, P.S. Sensitivity and specificity of immunohistochemical markers used in the diagnosis of epithelioid mesothelioma: A detailed systematic analysis using published data. Histopathology 2006, 48, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Sreekanth, K.V.; Alapan, Y.; ElKabbash, M.; Ilker, E.; Hinczewski, M.; Gurkan, U.A.; De Luca, A.; Strangi, G. Extreme sensitivity biosensing platform based on hyperbolic metamaterials. Nat. Mater. 2016, 15, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, S.P.; Qiu, G.; Ding, N.; Lu, X.; Wu, C.M.L. Label-free detection of 3-nitro-l-tyrosine with nickel-doped graphene localized surface plasmon resonance biosensor. Biosens. Bioelectron. 2017, 89, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Otto, L.M.; Mohr, D.A.; Johnson, T.W.; Oh, S.H.; Lindquist, N.C. Polarization interferometry for real-time spectroscopic plasmonic sensing. Nanoscale 2015, 7, 4226–4233. [Google Scholar] [CrossRef] [Green Version]
- Yesilkoy, F. Optical interrogation techniques for nanophotonic biochemical sensors. Sensors 2019, 19, 4287. [Google Scholar] [CrossRef] [Green Version]
- Piliarik, M.; Homola, J. Surface plasmon resonance (SPR) sensors: Approaching their limits? Opt. Express 2009, 17, 16505–16517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jefferson, M.; Hobbs, P.C.; Risk, W.P.; Feller, B.E.; Miller, R.D.; Knoesen, A. Shot-noise limited detection for surface plasmon sensing. Opt. Express 2011, 19, 107–117. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Kenney, M.; Su, X.; Xu, N.; Ouyang, C.; Shi, Y.; Han, J.; Zhang, W.; Zhang, S. Broadband metasurfaces with simultaneous control of phase and amplitude. Adv. Mater. 2014, 26, 5031–5036. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Tsai, W.-Y.; Chen, W.T.; Huang, Y.-W.; Chen, T.-Y.; Chen, J.-W.; Liao, C.Y.; Chu, C.H.; Sun, G.; Tsai, D.P. Versatile polarization generation with an aluminum plasmonic metasurface. Nano Lett. 2017, 17, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Genevet, P.; Kats, M.A.; Aieta, F.; Tetienne, J.-P.; Capasso, F.; Gaburro, Z. Light propagation with phase discontinuities: Generalized laws of reflection and refraction. Science 2011, 334, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Arbabi, A.; Arbabi, E.; Horie, Y.; Kamali, S.M.; Faraon, A. Planar metasurface retroreflector. Nat. Photonics 2017, 11, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wu, P.C.; Su, V.-C.; Lai, Y.-C.; Chu, C.H.; Chen, J.-W.; Lu, S.-H.; Chen, J.; Xu, B.; Kuan, C.-H. Broadband achromatic optical metasurface devices. Nat. Commun. 2017, 8, 187. [Google Scholar] [CrossRef]
- Mueller, J.B.; Rubin, N.A.; Devlin, R.C.; Groever, B.; Capasso, F. Metasurface polarization optics: Independent phase control of arbitrary orthogonal states of polarization. Phys. Rev. Lett. 2017, 118, 113901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, T.; Lee, S.-Y.; Song, E.Y.; Chun, H.; Lee, B. Plasmonic nanostructures for nano-scale bio-sensing. Sensors 2011, 11, 10907–10929. [Google Scholar] [CrossRef]
- Roh, S.; Chung, T.; Lee, B. Overview of the characteristics of micro- and nano-structured surface plasmon resonance sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Li, S.; Sun, H. Metamaterials application in sensing. Sensors 2012, 12, 2742–2765. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [Green Version]
- Zayats, A.V.; Smolyaninov, I.I. Near-field photonics: Surface plasmon polaritons and localized surface plasmons. J. Opt. A Pure Appl. Opt. 2003, 5, S16. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance-Based Sensors; Springer Science & Business Media: Berlin, Germany, 2006; Volume 4. [Google Scholar]
- Xu, Y.; Bai, P.; Zhou, X.; Akimov, Y.; Png, C.E.; Ang, L.K.; Knoll, W.; Wu, L. Optical refractive index sensors with plasmonic and photonic structures: Promising and inconvenient truth. Adv. Opt. Mater. 2019, 7, 1801433. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.; Lin, S.; Jiang, S.; Liu, X.; Guo, S. Low-cost flexible plasmonic nanobump metasurfaces for label-free sensing of serum tumor marker. Biosens. Bioelectron. 2020, 150, 111905. [Google Scholar] [CrossRef]
- Im, H.; Shao, H.; Park, Y.I.; Peterson, V.M.; Castro, C.M.; Weissleder, R.; Lee, H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat. Biotechnol. 2014, 32, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.J.; Sadhu, S.; Ray, S.; Srivastava, S. Cancer biomarker detection by surface plasmon resonance biosensors. Clin. Lab. Med. 2012, 32, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tao, F.; Zhu, J.; Lin, S.; Wang, Z.; Wang, X.; Ou, J.-Y.; Li, Y.; Liu, Q.H. Portable tumor biosensing of serum by plasmonic biochips in combination with nanoimprint and microfluidics. Nanophotonics 2019, 8, 307–316. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Slawin, K.M.; Brawer, M.K.; Flanigan, R.C.; Patel, A.; Richie, J.P.; DeKernion, J.B.; Walsh, P.C.; Scardino, P.T. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: A prospective multicenter clinical trial. JAMA 1998, 279, 1542–1547. [Google Scholar] [CrossRef]
- Damborska, D.; Bertok, T.; Dosekova, E.; Holazova, A.; Lorencova, L.; Kasak, P.; Tkac, J. Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta 2017, 184, 3049–3067. [Google Scholar] [CrossRef]
- Kim, W.T.; Yun, S.J.; Kim, W.-J. For physicians managing voiding dysfunction, improving the detection rate of early prostate cancer and discrimination from benign prostatic hyperplasia, in a molecular biomarker aspect. Int. Neurourol. J. 2019, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Li, A.; Chang, L.; Li, L.; Guo, L. Gold nano disks arrays for localized surface plasmon resonance-based detection of PSA cancer marker. Sens. Actuators B Chem. 2018, 255, 1298–1307. [Google Scholar] [CrossRef]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couture, M.; Ray, K.K.; Poirier-Richard, H.-P.; Crofton, A.; Masson, J.-F. 96-well plasmonic sensing with nanohole arrays. ACS Sens. 2016, 1, 287–294. [Google Scholar] [CrossRef]
- Li, W.; Qiu, Y.; Zhang, L.; Jiang, L.; Zhou, Z.; Chen, H.; Zhou, J. Aluminum nanopyramid array with tunable ultraviolet–visible–infrared wavelength plasmon resonances for rapid detection of carbohydrate antigen 199. Biosens. Bioelectron. 2016, 79, 500–507. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, J.; Liu, T.; Tao, Y.; Jiang, R.; Liu, M.; Xiao, G.; Zhu, J.; Zhou, Z.-K.; Wang, X. Plasmonic gold mushroom arrays with refractive index sensing figures of merit approaching the theoretical limit. Nat. Commun. 2013, 4, 2381. [Google Scholar] [CrossRef] [PubMed]
- Predabon, S.M.; Buzzetti, P.H.; Visentainer, J.E.; Visentainer, J.V.; Radovanovic, E.; Monteiro, J.P.; Girotto, E.M. Detection of tumor necrosis factor-alpha cytokine from the blood serum of a rat infected with Pb18 by a gold nanohole array-based plasmonic biosensor. J. Nanophotonics. 2020, 14, 036004. [Google Scholar] [CrossRef]
- Jiao, F.; Li, F.; Shen, J.; Guan, C.; Khan, S.A.; Wang, J.; Yang, Z.; Zhu, J. Wafer-Scale Flexible Plasmonic Metasurface with Passivated Aluminum Nanopillars for High-Sensitivity Immunosensors. Sens. Actuators B Chem. 2021, 344, 130170. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Chen, H. A route to terahertz metamaterial biosensor integrated with microfluidics for liver cancer biomarker testing in early stage. Sci. Rep. 2017, 7, 16378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Chin, L.K.; Son, T.; Hong, J.-S.; Liu, A.-Q.; Skog, J.; Castro, C.M.; Weissleder, R.; Lee, H.; Im, H. Plasmonic sensors for extracellular vesicle analysis: From scientific development to translational research. ACS Nano 2020, 14, 14528–14548. [Google Scholar] [CrossRef]
- Park, J.; Im, H.; Hong, S.; Castro, C.M.; Weissleder, R.; Lee, H. Analyses of intravesicular exosomal proteins using a nano-plasmonic system. ACS Photonics 2018, 5, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.S.; Im, H.; Hong, S.; Pergolini, I.; Del Castillo, A.F.; Wang, R.; Clardy, S.; Huang, C.-H.; Pille, C.; Ferrone, S. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci. Transl. Med. 2017, 9, eaal3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.Z.; Zhang, Y.; Chen, Y.; Zhao, H.; Stephenson, M.C.; Ho, N.R.; Chen, Y.; Chung, J.; Reilhac, A.; Loh, T.P. Subtyping of circulating exosome-bound amyloid β reflects brain plaque deposition. Nat. Commun. 2019, 10, 1144. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Cheng, L.; Hu, L.; Lou, D.; Zhang, T.; Li, J.; Zhu, Q.; Liu, F. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens. Bioelectron. 2020, 163, 112290. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Yang, M.; Pang, S.W. Highly sensitive detection of exosomes by 3D plasmonic photonic crystal biosensor. Nanoscale 2018, 10, 19927–19936. [Google Scholar] [CrossRef]
- Hackett, L.P.; Ameen, A.; Li, W.; Dar, F.K.; Goddard, L.L.; Liu, G.L. Spectrometer-free plasmonic biosensing with metal–insulator–metal nanocup arrays. ACS Sens. 2018, 3, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Yavas, O.; Svedendahl, M.; Dobosz, P.; Sanz, V.; Quidant, R. On-a-chip biosensing based on all-dielectric nanoresonators. Nano Lett. 2017, 17, 4421–4426. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Muruato, A.E.; Fontes-Garfias, C.R.; Ren, P.; Garcia-Blanco, M.A.; Menachery, V.D.; Xie, X.; Shi, P.-Y. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat. Commun. 2020, 11, 4059. [Google Scholar] [CrossRef]
- Ai, J.-W.; Zhang, H.-C.; Xu, T.; Wu, J.; Zhu, M.; Yu, Y.-Q.; Zhang, H.-Y.; Li, Y.; Zhou, X.; Shen, Z. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Baek, Y.H.; Um, J.; Antigua, K.J.C.; Park, J.-H.; Kim, Y.; Oh, S.; Kim, Y.-i.; Choi, W.-S.; Kim, S.G.; Jeong, J.H. Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes Infect. 2020, 9, 998–1007. [Google Scholar] [CrossRef] [Green Version]
- Haveri, A.; Smura, T.; Kuivanen, S.; Österlund, P.; Hepojoki, J.; Ikonen, N.; Pitkäpaasi, M.; Blomqvist, S.; Rönkkö, E.; Kantele, A. Serological and molecular findings during SARS-CoV-2 infection: The first case study in Finland, January to February 2020. Eurosurveillance 2020, 25, 2000266. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I. Trends and innovations in biosensors for COVID-19 mass testing. ChemBioChem 2020, 21, 2880. [Google Scholar] [CrossRef] [PubMed]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C. Diagnosing COVID-19: The disease and tools for detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [Green Version]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chen, H.Y.; Foo, S.Y.; Tan, Y.-J.; Goh, P.-Y.; Wee, S.H. Recombinant protein-based enzyme-linked immunosorbent assay and immunochromatographic tests for detection of immunoglobulin G antibodies to severe acute respiratory syndrome (SARS) coronavirus in SARS patients. Clin. Diagn. Lab. Immunol. 2004, 11, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Day, M. COVID-19: Identifying and isolating asymptomatic people helped eliminate virus in Italian village. BMJ Br. Med. J. 2020, 368, m1165. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Zhu, F.; Guo, F.; Yang, B.; Wang, T. Detection of antibodies against SARS-CoV-2 in patients with COVID-19. J. Med. Virol. 2020, 92, 1735–1738. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.-P.; Lin, R.T.; Renia, L.; Ng, L.F. Serological approaches for COVID-19: Epidemiologic perspective on surveillance and control. Front. Immun. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Tian, D.; Liu, Y.; Lin, Z.; Lyon, C.J.; Lai, W.; Fusco, D.; Drouin, A.; Yin, X.; Hu, T. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis. Biosens. Bioelectron. 2020, 164, 112316. [Google Scholar] [CrossRef]
- Baraniuk, C. COVID-19 antibody tests: A briefing. BMJ 2020, 369, m2284. [Google Scholar] [CrossRef]
- La Marca, A.; Capuzzo, M.; Paglia, T.; Roli, L.; Trenti, T.; Nelson, S.M. Testing for SARS-CoV-2 (COVID-19): A systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online 2020, 41, 483–499. [Google Scholar] [CrossRef]
- Krammer, F.; Simon, V. Serology assays to manage COVID-19. Science 2020, 368, 1060–1061. [Google Scholar] [CrossRef]
- Chu, D.K.; Pan, Y.; Cheng, S.M.; Hui, K.P.; Krishnan, P.; Liu, Y.; Ng, D.Y.; Wan, C.K.; Yang, P.; Wang, Q. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, T.; Hu, W.; Zhang, W.; Song, Z.; Wang, Y.; Chen, M.; Xu, H.; Liu, G.L. Protein binding kinetics quantification via coupled plasmonic-photonic resonance nanosensors in generic microplate reader. Biosens. Bioelectron. 2019, 142, 111494. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and challenges for biosensors and nanoscale analytical tools for pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R. Assay techniques and test development for COVID-19 diagnosis. ACS Cent Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R. Development of point-of-care biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef]
- Mavrikou, S.; Moschopoulou, G.; Tsekouras, V.; Kintzios, S. Development of a portable, ultra-rapid and ultra-sensitive cell-based biosensor for the direct detection of the SARS-CoV-2 S1 spike protein antigen. Sensors 2020, 20, 3121. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.V.; Zhao, J.; Liu, J.; Wang, X.; Biswas, S.; Hewlett, I. Current approaches for diagnosis of influenza virus infections in humans. Viruses 2016, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Han, H.; Liu, F.; Lv, Z.; Wu, K.; Liu, Y.; Feng, Y.; Zhu, C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin. Chim. Acta 2020, 505, 172–175. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A review on plasmonic and metamaterial based biosensing platforms for virus detection. Sens. Bio-Sens. Res. 2021, 33, 100429. [Google Scholar] [CrossRef]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Smyrlaki, I.; Ekman, M.; Lentini, A.; de Sousa, N.R.; Papanicolaou, N.; Vondracek, M.; Aarum, J.; Safari, H.; Muradrasoli, S.; Rothfuchs, A.G. Massive and rapid COVID-19 testing is feasible by extraction-free SARS-CoV-2 RT-PCR. Nat. Commun. 2020, 11, 4812. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A. Diagnostic accuracy of serological tests for COVID-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2020, 172, 112752. [Google Scholar] [CrossRef]
- Soh, J.H.; Chan, H.-M.; Ying, J.Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 2020, 30, 100831. [Google Scholar] [CrossRef]
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Dai, Y.; Xu, Y.; Cai, Y.; Chen, X. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur. J. Clin. Microbiol. 2020, 39, 2271–2277. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souf, S. Recent advances in diagnostic testing for viral infections. Biosci. Horiz. Int. J. Stud. Res. 2016, 9, hzw010. [Google Scholar]
- Soler, M.; Estevez, M.C.; Cardenosa-Rubio, M.; Astua, A.; Lechuga, L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020, 5, 2663–2678. [Google Scholar] [CrossRef]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Lopez, G.A.; Estevez, M.-C.; Soler, M.; Lechuga, L.M. Recent advances in nanoplasmonic biosensors: Applications and lab-on-a-chip integration. Nanophotonics 2017, 6, 123–136. [Google Scholar] [CrossRef]
- Huang, L.; Ding, L.; Zhou, J.; Chen, S.; Chen, F.; Zhao, C.; Xu, J.; Hu, W.; Ji, J.; Xu, H. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 2021, 171, 112685. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Chu, K.-Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef]
- Yanik, A.A.; Huang, M.; Kamohara, O.; Artar, A.; Geisbert, T.W.; Connor, J.H.; Altug, H. An optofluidic nanoplasmonic biosensor for direct detection of live viruses from biological media. Nano Lett. 2010, 10, 4962–4969. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Gai, Z.; Saleh, L.; Tang, J.; Gui, T.; Kullak-Ublick, G.A.; Wang, J. Thermoplasmonic-assisted cyclic cleavage amplification for self-validating plasmonic detection of SARS-CoV-2. ACS Nano 2021, 15, 7536–7546. [Google Scholar] [CrossRef]
- Das, C.M.; Guo, Y.; Yang, G.; Kang, L.; Xu, G.; Ho, H.P.; Yong, K.T. Gold Nanorod Assisted Enhanced Plasmonic Detection Scheme of COVID-19 SARS-CoV-2 Spike Protein. Adv. Theor. Simul. 2020, 3, 2000185. [Google Scholar] [CrossRef] [PubMed]
- Moitra, P.; Alafeef, M.; Dighe, K.; Frieman, M.B.; Pan, D. Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 2020, 14, 7617–7627. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z.; Kaushik, A.; Manickam, P.; Ghoreishi, S.A. Functionalized terahertz plasmonic metasensors: Femtomolar-level detection of SARS-CoV-2 spike proteins. Biosens. Bioelectron. 2021, 177, 112971. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.; Yu, H.; Lee, C.Y.; Lee, J.-u.; Choi, H.-J.; Lee, J.-H.; Lee, H.; Cheon, J. Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nat. Biomed. Eng. 2020, 4, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, Y.; Wang, C.; Qiang, L.; Gao, J.; Wang, Y.; Liu, H.; Han, L.; Zhang, Y. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus specific genes through interaction between genes and nanoparticles. Anal. Chim. Acta 2021, 1154, 338330. [Google Scholar] [CrossRef]
- Hesselink, L.; Padhy, P.; Zaman, M.A.; Wu, M.; Jensen, M.A. In Trapping and manipulation on a chip: From subwavelength particle manipulation to chemical synthesis. In Proceedings of the Optical Trapping and Optical Micromanipulation XVIII, San Diego, CA, USA, 1–5 August 2021; p. 117981P. [Google Scholar]
- Zaman, M.A.; Padhy, P.; Hesselink, L. Near-field optical trapping in a non-conservative force field. Sci. Rep. 2019, 9, 649. [Google Scholar] [CrossRef]
- Padhy, P.; Zaman, M.A.; Hesselink, L. In-plane near-field optical barrier on a chip. Opt. Lett. 2019, 44, 2061–2064. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Hesselink, L. Fokker-Planck analysis of optical near-field traps. Sci. Rep. 2019, 9, 9557. [Google Scholar] [CrossRef]
- Wen, D.; Crozier, K.B. Metasurfaces 2.0: Laser-integrated and with vector field control. APL Photonics 2021, 6, 080902. [Google Scholar] [CrossRef]
- Ye, M.; Zha, J.; Tan, C.; Crozier, K.B. Graphene-based mid-infrared photodetectors using metamaterials and related concepts. Appl. Phys. Rev. 2021, 8, 031303. [Google Scholar] [CrossRef]
- Li, N.; Cadusch, J.; Liu, A.; Barlow, A.J.; Roberts, A.; Crozier, K.B. Algorithm-Designed Plasmonic Nanotweezers: Quantitative Comparison by Theory, Cathodoluminescence, and Nanoparticle Trapping. Adv. Opt. Mater. 2021, 9, 2100758. [Google Scholar] [CrossRef]
- Oh, S.H.; Altug, H.; Jin, X.; Low, T.; Koester, S.J.; Ivanov, A.P.; Edel, J.B.; Avouris, P.; Strano, M.S. Nanophotonic biosensors harnessing van der Waals materials. Nat. Commun. 2021, 12, 3824. [Google Scholar] [CrossRef]
- Wang, X.; Jian, J.; Diaz-Amaya, S.; Kumah, C.E.; Lu, P.; Huang, J.; Lim, D.G.; Pol, V.G.; Youngblood, J.P.; Boltasseva, A. Hybrid plasmonic Au–TiN vertically aligned nanocomposites: A nanoscale platform towards tunable optical sensing. Nanoscale Adv. 2019, 1, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, S.M.; Wang, D.; Chaudhuri, K.; DeVault, C.; Kildishev, A.V.; Boltasseva, A.; Shalaev, V.M. Material platforms for optical metasurfaces. Nanophotonics 2018, 7, 959–987. [Google Scholar] [CrossRef]
- Guo, W.-P.; Mishra, R.; Cheng, C.-W.; Wu, B.-H.; Chen, L.-J.; Lin, M.-T.; Gwo, S. Titanium nitride epitaxial films as a plasmonic material platform: Alternative to gold. ACS Photonics 2019, 6, 1848–1854. [Google Scholar] [CrossRef]
- Rastgordani, A.; Kashani, Z.G. Robust design method for metasurface high-sensitivity sensors and absorbers. J. Opt. Soc. Am. B 2020, 37, 2006–2011. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Mao, Y.F.; Chen, J.; Wang, S.; Chen, H.Z.; Zhang, Y.; Wang, S.Y.; Chen, X.; Li, T.; et al. Stable, high-performance sodium-based plasmonic devices in the near infrared. Nature 2020, 581, 401–405. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Tittl, A.; Yue, S.; Giessen, H.; Song, C.; Ding, B.; Liu, N. DNA-assembled bimetallic plasmonic nanosensors. Light Sci. Appl. 2014, 3, e226. [Google Scholar] [CrossRef] [Green Version]
- Malkiel, I.; Mrejen, M.; Nagler, A.; Arieli, U.; Wolf, L.; Suchowski, H. Plasmonic nanostructure design and characterization via deep learning. Light Sci. Appl. 2018, 7, 8074. [Google Scholar] [CrossRef]
- Li, X.; Shu, J.; Gu, W.; Gao, L. Deep neural network for plasmonic sensor modeling. Opt. Mater. Express 2019, 9, 3857–3862. [Google Scholar] [CrossRef]
- Tittl, A.; John-Herpin, A.; Leitis, A.; Arvelo, E.R.; Altug, H. Metasurface-based molecular biosensing aided by artificial intelligence. Angew. Chem. Int. Ed. 2019, 58, 14810–14822. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.C.M.; Souza, L.C.; Oliveira, L.C. SmartSPR sensor: Machine learning approaches to create intelligent surface plasmon based sensors. Biosens. Bioelectron. 2021, 172, 112760. [Google Scholar] [CrossRef] [PubMed]

| Metastructure | Analytes | Bulk Sensitivity | LOD | Reference |
|---|---|---|---|---|
| Nanohole | CEA | 490.2 nm/RIU | 5 ng/mL | [79] |
| Nanopillar | CEA | 454.4 nm/RIU | 5 ng/mL | [76] |
| Nanocup | CEA | 800 ΔT%/RIU | 10 ng/mL | [100] |
| Nanodisk | PSA | 113 nm/RIU | 1.49 ng/mL | [83] |
| Nonohole | PSA | / | 0.1 nM | [86] |
| Nanohole | CD24 | / | 0.18 ng/μL | [77] |
| Nanopyramid | CA199 | 819 nm/RIU | 29 ng/mL | [87] |
| Nanomushroom | AFP | 1015 nm/RIU | 15 ng/mL | [88] |
| Nanosplit-ring | AFP | / | 0.02524 μg/mL | [91] |
| Nanohole | TNF-α | 4000–5300 IU/RIU | 17 pg/mL | [89] |
| Nanohole | Aβ | / | 200 exosomes | [97] |
| Nanoporosity | CD-63 | / | 1 particle/μL | [98] |
| Nanohole | Exosomes | 1736 nm/RIU | / | [99] |
| Nanopillar | CEA | / | 5 ng/mL | [40] |
| Nanodisk | PSA | / | 1.6 ng/mL | [101] |
| Metastructure | Analytes | LOD | Reference |
|---|---|---|---|
| Nanospike | S protein | 0.08 ng/mL | [141] |
| Nanocup | S protein | 370 vp/mL | [140] |
| Nanoisland | SARS-CoV-2 | 0.22 pM | [143,144] |
| Nanorod | S protein | 111.11 deg/RIU | [145] |
| Nanoparticle | N gene | 0.18 ng/uL | [146] |
| Toroidal metasurface/nanoparticle | S protein | 4.2 fM | [147] |
| Nanohole | S protein | / | [142] |
| Nanoparticle | RNA | 160 fM | [149] |
| Nanoparticle | RNA | 3.2 gene/uL | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, J.; Khan, S.A.; Li, F.; Shen, J.; Duan, Q.; Liu, X.; Zhu, J. Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review. Sensors 2022, 22, 133. https://doi.org/10.3390/s22010133
Wang Z, Chen J, Khan SA, Li F, Shen J, Duan Q, Liu X, Zhu J. Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review. Sensors. 2022; 22(1):133. https://doi.org/10.3390/s22010133
Chicago/Turabian StyleWang, Zhenbiao, Junjie Chen, Sayed Ali Khan, Fajun Li, Jiaqing Shen, Qilin Duan, Xueying Liu, and Jinfeng Zhu. 2022. "Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review" Sensors 22, no. 1: 133. https://doi.org/10.3390/s22010133
APA StyleWang, Z., Chen, J., Khan, S. A., Li, F., Shen, J., Duan, Q., Liu, X., & Zhu, J. (2022). Plasmonic Metasurfaces for Medical Diagnosis Applications: A Review. Sensors, 22(1), 133. https://doi.org/10.3390/s22010133






