1. Introduction
Phenethylamine, a trace amine and monoamine alkaloid, acts as a neuromodulator in the human central nervous system [
1]. Dysregulation of phenethylamine levels has been implicated in psychiatric and neurological disorders, including depression, schizophrenia, and attention-deficit/hyperactivity disorder (ADHD) [
2]. In medicinal chemistry, phenethylamine derivatives are incorporated into pharmaceuticals and exhibit potent antioxidant, antibacterial, and anticancer properties [
3]. These compounds demonstrate selective cytotoxicity, sparing healthy cells while effectively targeting malignant cells and pathogenic bacteria [
1].
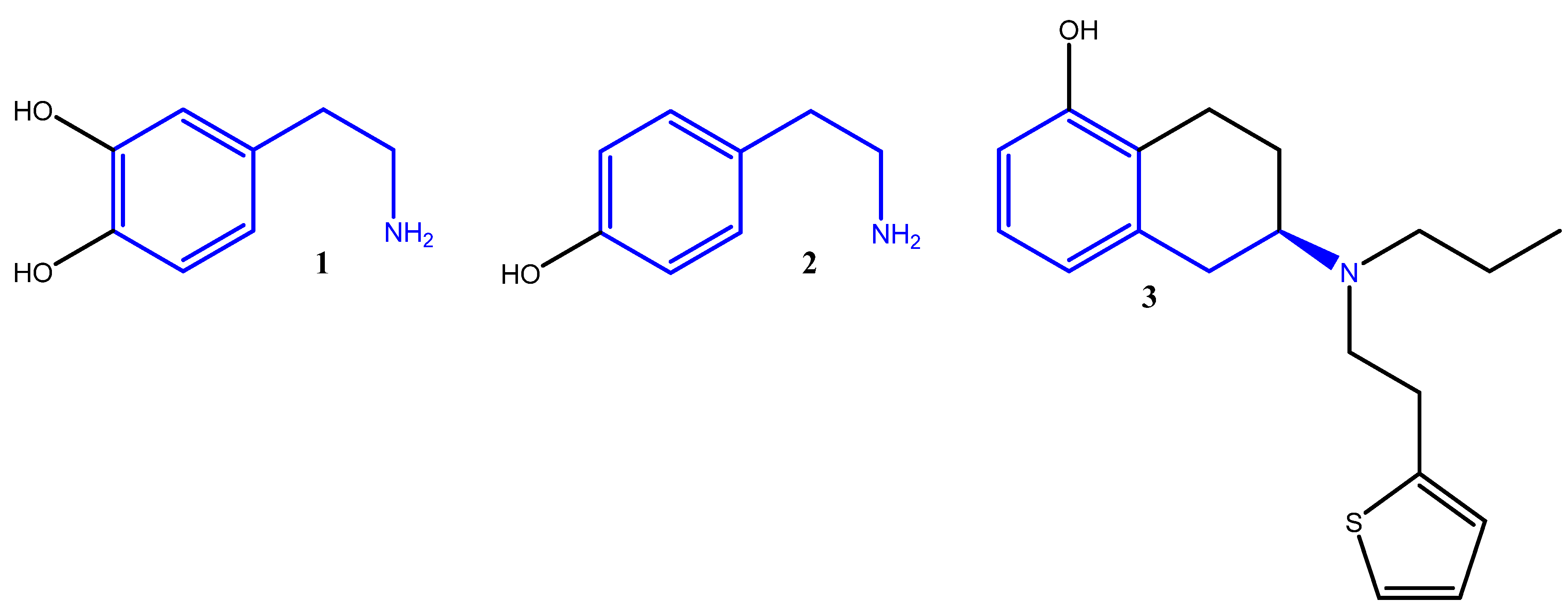
Primary amines, particularly substituted phenethylamines such as dopamine
1 and tyramine
2, are important in agrochemical and pharmaceutical applications due to their significant biological roles. Dopamine (3,4-dihydroxyphenethylamine) is a key neurotransmitter involved in motor control [
4], while tyramine (4-hydroxyphenethylamine), a biogenic amine found in fermented foods, participates in metabolic processes [
5]. Structurally related to dopamine, tyramine is a biologically active trace amine that modulates monoaminergic neurotransmission [
6], contributing to its relevance in neurological [
7] and pharmacological [
8] research. Additionally, rotigotine
3, a 2-aminotetralin derivative marketed as Neupro
4, is used to treat Parkinson’s disease and restless leg syndrome (
Figure 1) [
9].
Valproic acid is a widely prescribed drug recognized for its strong pharmacodynamic profile and broad therapeutic applicability. Clinically, it is used primarily in the treatment of epilepsy, migraine prevention, and mood stabilization in disorders such as bipolar disorder, anxiety, and other psychiatric conditions [
10]. Its effectiveness against a wide range of seizure types has supported its extensive global use for more than four decades. Importantly, valproic acid acts as a histone deacetylase inhibitor, a property that has prompted investigation into its potential as an adjunct therapy in oncology, HIV treatment, and neurodegenerative diseases. However, exposure during early pregnancy is associated with an increased risk of autism spectrum disorders in offspring, underscoring a critical safety concern [
11,
12].
Pharmaceuticals and other chemicals frequently have amide functionality, which is essential to organic chemistry. Since amides are involved in a large portion of pharmaceutical processes, sustainable synthesis techniques that optimize atom efficiency and reduce waste are desperately needed [
13].
Considering the significant biological relevance of both valproic acid and biogenic 2-phenylethylamines, the synthesis of new hybrid molecules combining these two pharmacophores is of considerable interest for the investigation of their potential biological properties [
14,
15]. The rational conjugation of valproic acid with substituted phenethylamines such as 2-(4-methoxyphenyl)ethan-1-amine may lead to hybrid compounds with enhanced or synergistic pharmacological profiles, potentially improving therapeutic efficacy or selectivity. The incorporation of amide functionality in such hybrids aligns with the prevalence of amide bonds in pharmaceuticals and supports the development of structurally robust molecules. Therefore, these hybrid systems represent promising candidates for exploring new therapeutic avenues in neurological, psychiatric, and oncological research.
Based on the above, we report the successful synthesis of new hybrid molecule N-(4-methoxyphenethyl)-2-propylpentanamide, combining a valproic acid with 2-(4-methoxyphenyl)ethan-1-amine. The synthesis of the hybrid molecule was accomplished in two sequential steps: first, valproic acid was converted into the corresponding acyl chloride, followed by a green chemistry approach, employing a solvent-free methodology under mechanical force, thereby avoiding the use of organic solvents and aligning with sustainable and environmentally friendly synthetic practices.
2. Results and Discussion
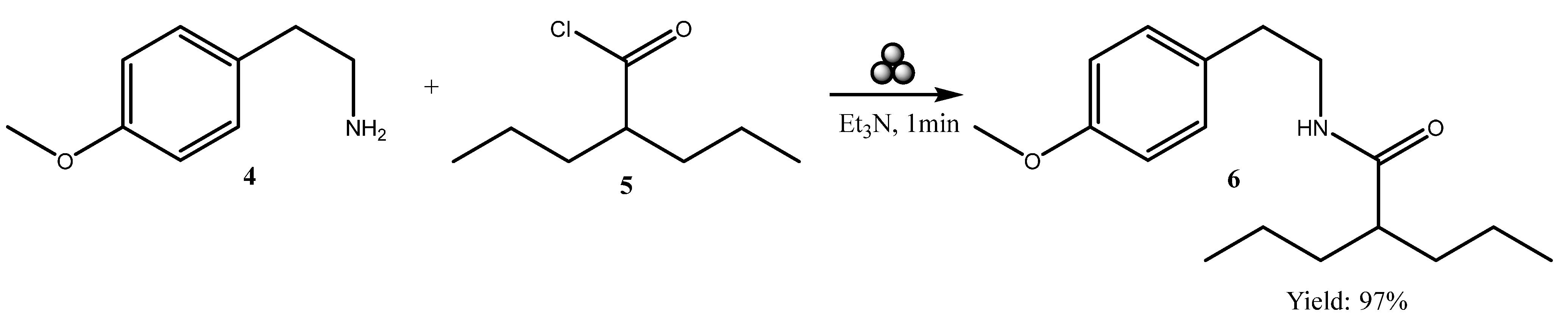
In this article, we report the use of mechanosynthesis for the preparation of
N-(4-methoxyphenethyl)-2-propylpentanamide. The procedure involved the activation of valproic acid via conversion to its acyl chloride, followed by an amidation with 2-(4-methoxyphenyl)ethan-1-amine under ball-milling conditions (
Scheme 1), in alignment with green chemistry principles.
By using mechanical force to forge this new hybrid molecule, our strategy exemplifies “medicinal mechanochemistry,” aiming to generate a multifunctional compound with potential applications in neurological and oncological therapeutics while adhering to environmentally responsible synthetic practices.
Analyzing the
1H NMR spectrum (
Figure S1), it can be observed that the compound is pure. All signals correspond to the protons in the molecule. A triplet integrating for 6 protons is observed at 0.74 ppm, corresponding to the two methyl groups of the valproic acid fragment. The next multiplet in the range 1.08–1.02 ppm, integrating for 4 protons, corresponds to the methylene groups adjacent to the methyl groups. The protons of the other two methylene groups adjacent to the methine group are stereotopic and appear as two separate multiplets, each integrating for 2 protons. The proton on the tertiary carbon is visualized as a multiplet in the range 2.05–1.98 ppm. The methylene group adjacent to the benzene ring appears as a triplet at 2.56 ppm, while the neighboring methylene group gives the next signal—a quartet for 2 protons at 3.18 ppm. The methoxy group appears as a singlet for 3 protons at 3.64 ppm. The aromatic protons appear as two doublets at 7.04 and 6.77 ppm, each integrating for 2 protons. The NH signal is a triplet observed at 7.75 ppm. The well-resolved signals unambiguously confirm the structure of the new hybrid molecule. Examining the
13C NMR spectrum (
Figure S2), 12 signals corresponding to the carbon atoms of the molecule can be observed. This provides further confirmation of the molecular structure. The IR (
Figure S3) and mass (
Figure S4) spectrometry data further unambiguously confirm the presence of the expected functional groups in compound 6, as well as the molecular weight of the newly synthesized hybrid molecule, reported here for the first time.
The structure of the synthesized amide was confirmed using a combination of analytical techniques, including melting-point determination, 1H and 13C NMR spectroscopy, infrared (IR) spectroscopy, and high-resolution mass spectrometry, together validating the successful formation and high purity of the target compound.
3. Materials and Methods
All reagents and chemicals were purchased from commercial suppliers (Sigma-Aldrich S.A., St. Louis, MO, USA, and Riedel-de Haën, Sofia, Bulgaria) and were used without further purification. Mechanosynthesis was conducted using a Retsch Planetary Ball Mill PM 200 (University of Plovdiv; RETSCH, Haan, Germany) equipped with a 25 mL stainless steel grinding jar. The grinding media consisted of 28 g of 12 mm hardened steel grinding balls. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance II + 600 spectrometer (BAS-IOCCP—Sofia, Bruker, Billerica, MA, USA), operating at 600 MHz for 1H and 150.9 MHz for 13C. Spectra were acquired in DMSO-d6, with chemical shifts (δ) referenced to tetramethylsilane (TMS, δ = 0.00 ppm) and coupling constants (J) reported in hertz (Hz). All NMR measurements were performed at ambient temperature (approximately 295 K). Melting points were determined using a Boetius hot-stage apparatus (University of Plovdiv; Boetius, Germany) and are reported uncorrected. Infrared (IR) spectra were obtained on a Bruker Alpha II FT-IR spectrometer (University of Plovdiv; Bruker, Billerica, MA, USA). High-resolution mass spectrometry (HRMS) analyses were conducted using a Q Exactive Plus mass spectrometer equipped with a heated electrospray ionization (HESI-II) source (Thermo Fisher Scientific, Bremen, Germany) and coupled to a Dionex Ultimate 3000RSLC UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA). Thin-layer chromatography (TLC) was carried out on 0.2 mm silica gel 60 plates (Fluka, Merck KGaA, Darmstadt, Germany).
3.1. Synthetic Procedures
3.1.1. Synthesis of 2-Propylpentanoyl chloride 5
Valproic acid (1.0 mmol, 0.1442 g) was dissolved in toluene (20 mL), and an excess of thionyl chloride (1.2 mmol, 0.087 mL) was added. The reaction mixture was refluxed at 110–115 °C for 2 h to convert the carboxylic acid into the corresponding acid chloride. After completion, the volatile components and excess toluene were removed under reduced pressure using a rotary evaporator. The resulting residue was dissolved in a small volume of dichloromethane and used directly in the subsequent reaction without further purification.
3.1.2. Synthesis of N-(4-Methoxyphenethyl)-2-propylpentanamide 6
4-Methoxyphenylethylamine 4 (1 mmol, 0.1512 g), 2-propylpentanoyl chloride (1 mmol, 0.1626 g), and Et3N (0.168 mL, 1.2 mmol) were placed in a 25 mL stainless steel grinding jar, along with four 12 mm hardened steel grinding balls. Mechanochemical reaction was performed in a Retsch PM 200 planetary ball mill with constant rotation of planetary (650 rpm) for 1 min. Progress of the reaction was monitored by thin-layer chromatography (petroleum ether/diethyl ether 1:1 (v/v)), confirming complete consumption of the amine starting material. Subsequent to that, the milling jar contents underwent a double wash with dichloromethane (10 mL) and water (5 mL) each, leading to the separation of the two layers. The aqueous layer was then subjected to extraction with dichloromethane (2 × 10 mL), and the combined organic fractions were washed with dilute aqueous hydrochloric acid (HCl:H2O = 1:4), saturated aqueous sodium carbonate solution, and brine. The organic phase was separated, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was then filtered throughout short chromatography column on neutral aluminum oxide using CH2Cl2 as eluent to afford the new hybrid molecule 6.
N-(4-Methoxyphenethyl)-2-propylpentanamide 6
White solid (m.p. 103–104 °C), yield 97% (0.2701 g), Rf = 0.51 (petroleum/diethyl ether = 1/1 v/v), 1H NMR (600 MHz, DMSO) δ 7.75 (t, J = 5.7 Hz, 1H), 7.04 (d, J = 8.9 Hz, 2H), 6.77 (d, J = 8.9 Hz, 2H), 3.64 (s, 3H), 3.18 (q, J = 12.8, 7.3 Hz, 2H), 2.56 (t, J = 7.3 Hz, 2H), 2.05–1.98 (m, 1H), 1.40–1.28 (m, 2H), 1.15–1.09 (m, 2H), 1.08–1.02 (m, 4H), 0.74 (t, J = 7.2 Hz, 6H). 13C NMR (151 MHz, DMSO) δ 175.13 (C=O), 158.10 (CH3OCsp2), 131.85 (CH2Csp2), 130.03 (Csp2H), 114.10 (Csp2H), 55.42 (CH3O), 45.71 (CH2CH(C=O)CH2), 40.57 (CH2NH), 35.35 (CH2CH2NH), 34.88 (CHCH2CH2CH3), 20.62 (CHCH2CH2CH3), 14.46 (CHCH2CH2CH3). Electrospray ionization (ESI) m/z calculated for [M + Na]+ C17H27NNaO2+ = 300.1934, found 300.1925 (mass error ∆m = −3.00 ppm). IR (KBr) νmax., cm−1: 3293 ν(N-H), 2954 νas (CH3), 2930 νas (CH2), 2869 νs (CH3), 1641, 1614 ν (C=O), 1457 δas (CH3), 1380 δs (CH3).