(Z)-8-Hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione
Abstract
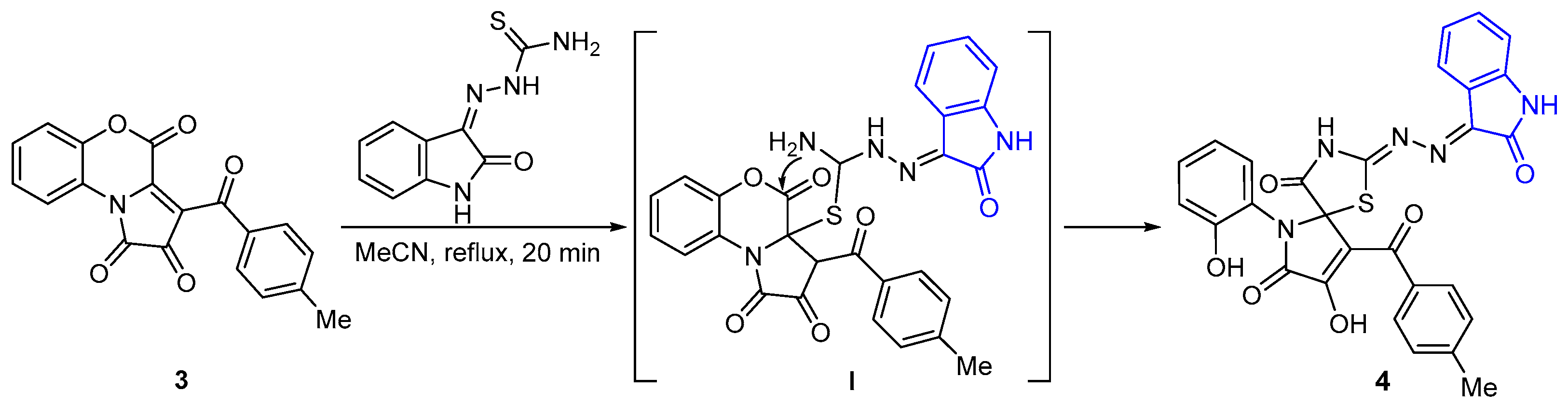
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Information
3.2. Preparation of the Title Compound
3.3. X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khetmalis, Y.M.; Shivani, M.; Murugesan, S.; Chandra Sekhar, K.V.G. Oxindole and Its Derivatives: A Review on Recent Progress in Biological Activities. Biomed. Pharmacother. 2021, 141, 111842. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Monga, Y.; Gupta, A.; Singh, S. 2-Oxindole and Related Heterocycles: Synthetic Methodologies for Their Natural Products and Related Derivatives. RSC Adv. 2023, 13, 14249–14267. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Hayallah, A.M.; Bukhari, S.N.A.; Musa, A.; Elmowafy, M.; Abdel-Rahman, H.M.; Abd El-Gaber, M.K. Design, Synthesis, Molecular Modeling, and Anticancer Evaluation of New VEGFR-2 Inhibitors Based on the Indolin-2-One Scaffold. Pharmaceuticals 2022, 15, 1416. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Hashemi, M.; Eskandarpour, V.; Zarghi, A.; Hadizadeh, F.; Ghodsi, R. New Indolin-2-Ones, Possessing Sunitinib Scaffold as HDAC Inhibitors and Anti-Cancer Agents with Potential VEGFR Inhibition Activity; Design, Synthesis and Biological Evaluation. Bioorg. Chem. 2025, 156, 108231. [Google Scholar] [CrossRef] [PubMed]
- Jhagta, C.; Singh, M. Oxindole Derivatives as Multi-Target Agents for Alzheimer’s Disease: A Promising Therapeutic Strategy. ChemistrySelect 2025, 10, e00743. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, S.; Chen, W.; Du, T.; Wang, D.; Wang, Z.; Chen, J. Recent Advances and Prospects of Indole Derivatives in the Discovery of New Agricultural Chemicals. J. Agric. Food Chem. 2025, 73, 22979–22993. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, X.; Shi, S.; Bai, L.; Hu, D.; Song, R.; Song, B. 3-Hydroxy-2-Oxindole Derivatives Containing Sulfonamide Motif: Synthesis, Antiviral Activity, and Modes of Action. J. Agric. Food Chem. 2023, 71, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.W.; Krushinski, J.H.; Beedle, E.E.; Wyss, V.; Pollock, G.D.; Wilson, H.; Kauffman, R.F.; Hayes, J.S. Dihydropyridazinone Cardiotonics. The Discovery and Inotropic Activity of 1,3-Dihydro-3,3-Dimethyl-5-(1,4,5,6-Tetrahydro-6-Oxo-3-Pyridazinyl)-2H-Indol-2-One. J. Med. Chem. 1986, 29, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Sethi, K.D.; Hauser, R.A.; Davis, T.L.; Hammerstad, J.P.; Bertoni, J.; Taylor, R.L.; Sanchez-Ramos, J.; O’Brien, C.F.; Ropinirole Study Group. Ropinirole for the Treatment of Early Parkinson’s Disease. Neurology 1997, 49, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Jia, Y.; Shen, Y.; He, H.; Fang, R.; Chen, X.; Hao, X. 3-Acetonyl-3-hydroxyoxindole: A New Inducer of Systemic Acquired Resistance in Plants. Plant Biotechnol. J. 2008, 6, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, X. Natural Product Sciences: An Integrative Approach to the Innovations of Plant Natural Products. Sci. China Life Sci. 2020, 63, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.D.; Zardo, R.S.; Figueiredo, G.S.M.; Silva, B.V.; Pinto, A.C. Anti-Inflammatory Properties of Convolutamydine A and Two Structural Analogues. Life Sci. 2014, 116, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lukmanova, D.N.; Prikhod’ko, Y.I.; Dmitriev, M.V.; Mashevskaya, I.V.; Maslivets, A.N. Synthesis of Spiro[Pyrrole-2,5′-Thiazoles] by Heterocyclization of Pyrrolobenzoxazinetriones with Aromatic Aldehyde Thiosemicarbazones. Russ. J. Org. Chem. 2019, 55, 108–114. [Google Scholar] [CrossRef]
- Lukmanova, D.N.; Balandina, S.Y.; Makhmudov, R.R.; Mashevskaya, I.V. Antinociceptive and Antimicrobial Activity of Products from Reactions of Pyrrolobenzoxazinetriones with Thiosemicarbazones of Aromatic and Heteroaromatic Aldehydes. Pharm. Chem. J. 2020, 54, 236–240. [Google Scholar] [CrossRef]
- Maslivets, A.N.; Mashevskaya, I.V.; Krasnykh, O.P.; Shurov, S.N.; Andreichikov, Y.S. 5-membered 2, 3-dioxoheterocycles. 33. Synthesis of 3-aroyl-1,2-dihydro-4H-pyrrolo-[5.1-c][1,4]benzoxazine-1,2,4-triones and their interaction with water and alcohols. Zhurn. Org. Khim 1992, 28, 2545–2553. [Google Scholar]
- Hernándeza, W.; Paz, J.; Vaisberg, A.; Spodine, E.; Richter, R.; Beyer, L. Synthesis, Characterization, and In Vitro Cytotoxic Activities of Benzaldehyde Thiosemicarbazone Derivatives and Their Palladium (II) and Platinum (II) Complexes against Various Human Tumor Cell Lines. Bioinorg. Chem. Appl. 2008, 2008, 690952. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, Version 1.171.37.33; Agilent Technologies: Wroclaw, Poland, 2014. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 16 November 2025).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]




| D–H⋯A | D–H, Å | H⋯A, Å | D⋯A, Å | Angle D–H⋯A, ° |
|---|---|---|---|---|
| N1—H1⋯O1W * | 0.89 (3) | 1.81 (3) | 2.695 (4) | 173 (3) |
| O3—H3⋯O1 ** | 0.88 (3) | 1.80 (4) | 2.637 (2) | 159 (3) |
| O4—H4⋯O2S | 0.97 (4) | 1.74 (4) | 2.699 (11) | 170 (4) |
| N5—H5⋯O1S | 0.81 (3) | 2.03 (3) | 2.840 (11) | 174 (3) |
| O1W—H1WA⋯O2S | 0.82 (2) | 2.19 (4) | 2.908 (12) | 146 (6) |
| O1W—H1WB⋯O1S *** | 0.83 (2) | 1.87 (3) | 2.683 (16) | 165 (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Belozerova, D.N.; Dmitriev, M.V.; Mashevskaya, I.V. (Z)-8-Hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione. Molbank 2026, 2026, M2116. https://doi.org/10.3390/M2116
Belozerova DN, Dmitriev MV, Mashevskaya IV. (Z)-8-Hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione. Molbank. 2026; 2026(1):M2116. https://doi.org/10.3390/M2116
Chicago/Turabian StyleBelozerova, Dzhamilia N., Maksim V. Dmitriev, and Irina V. Mashevskaya. 2026. "(Z)-8-Hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione" Molbank 2026, no. 1: M2116. https://doi.org/10.3390/M2116
APA StyleBelozerova, D. N., Dmitriev, M. V., & Mashevskaya, I. V. (2026). (Z)-8-Hydroxy-6-(2-hydroxyphenyl)-9-(4-methylbenzoyl)-2-(((E)-2-oxoindolin-3-ylidene)hydrazineylidene)-1-thia-3,6-diazaspiro[4.4]non-8-ene-4,7-dione. Molbank, 2026(1), M2116. https://doi.org/10.3390/M2116





