2-(Difluoroboryloxy)benzamide
Abstract
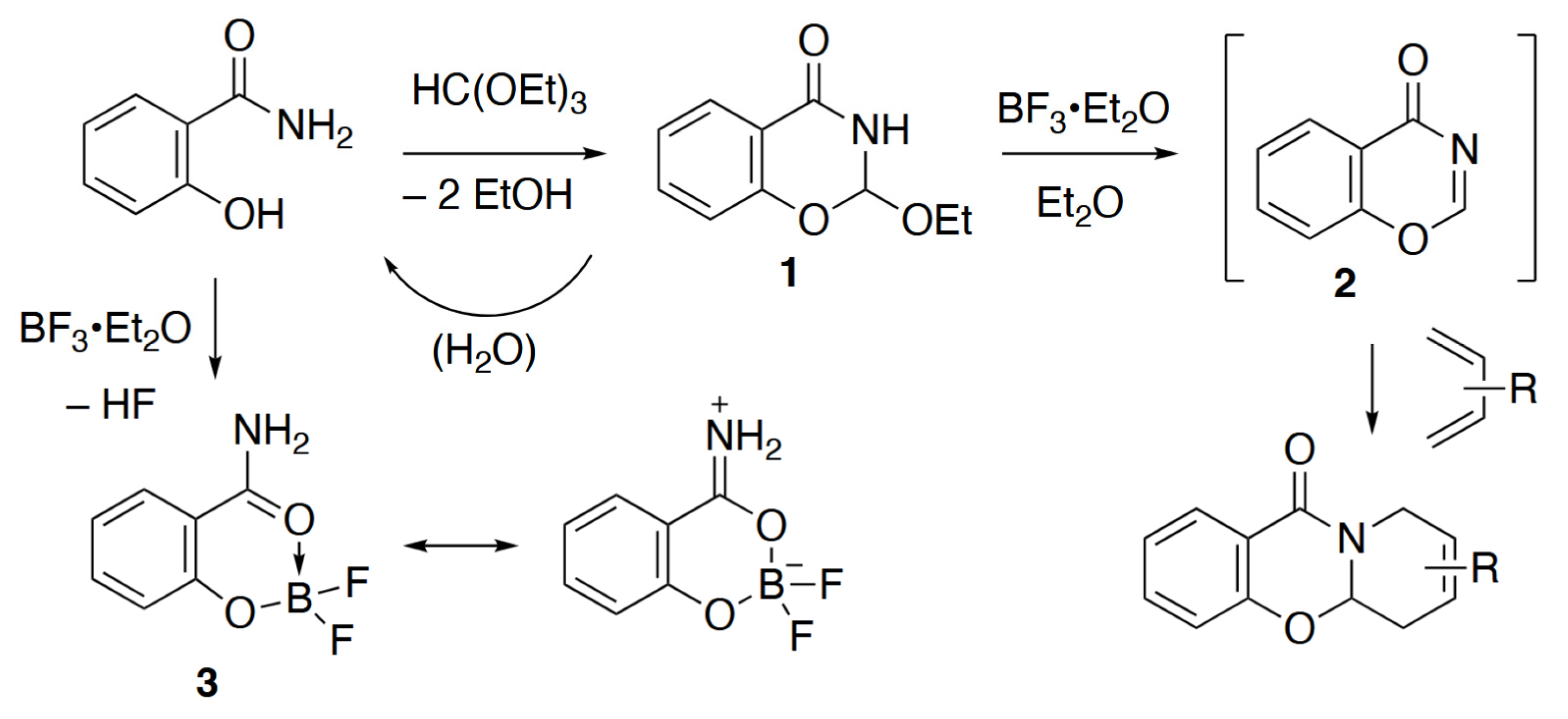
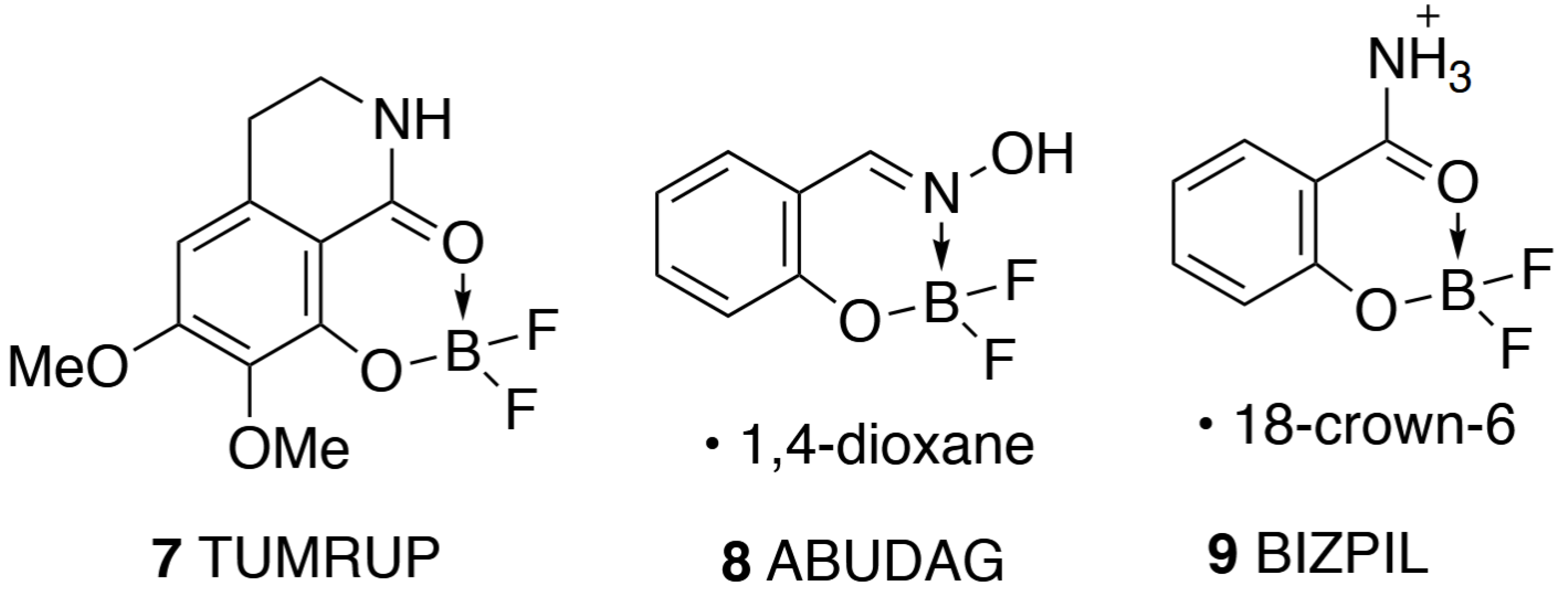
1. Introduction
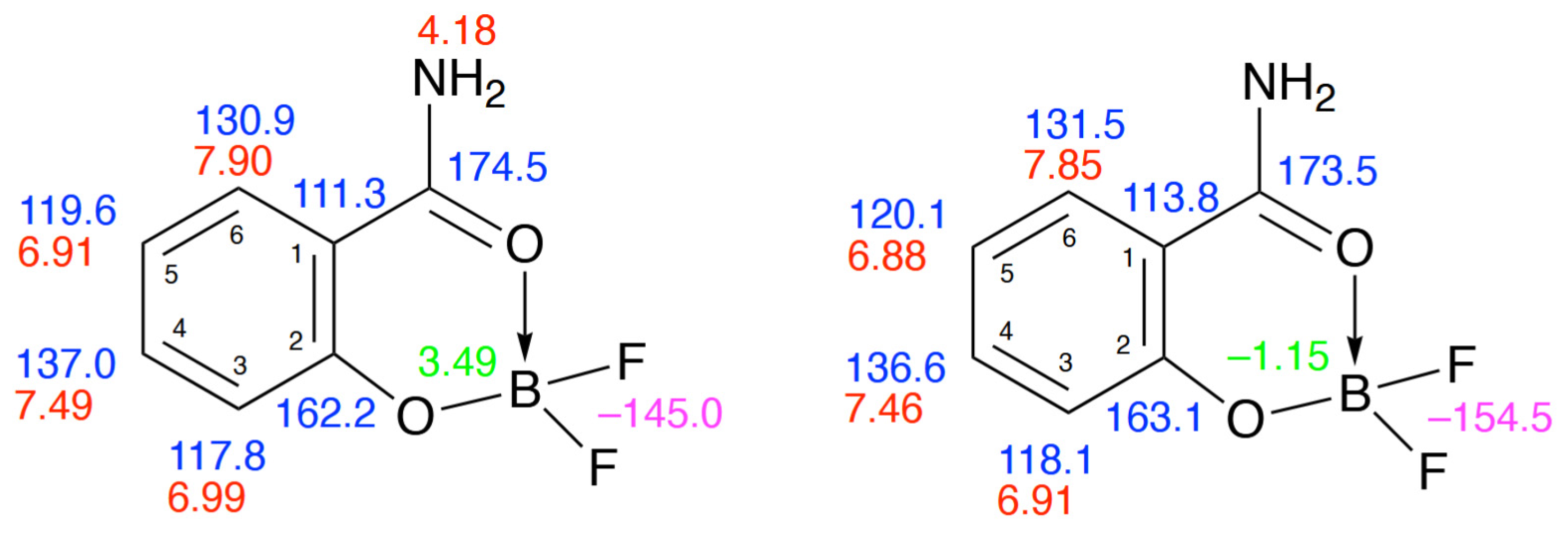
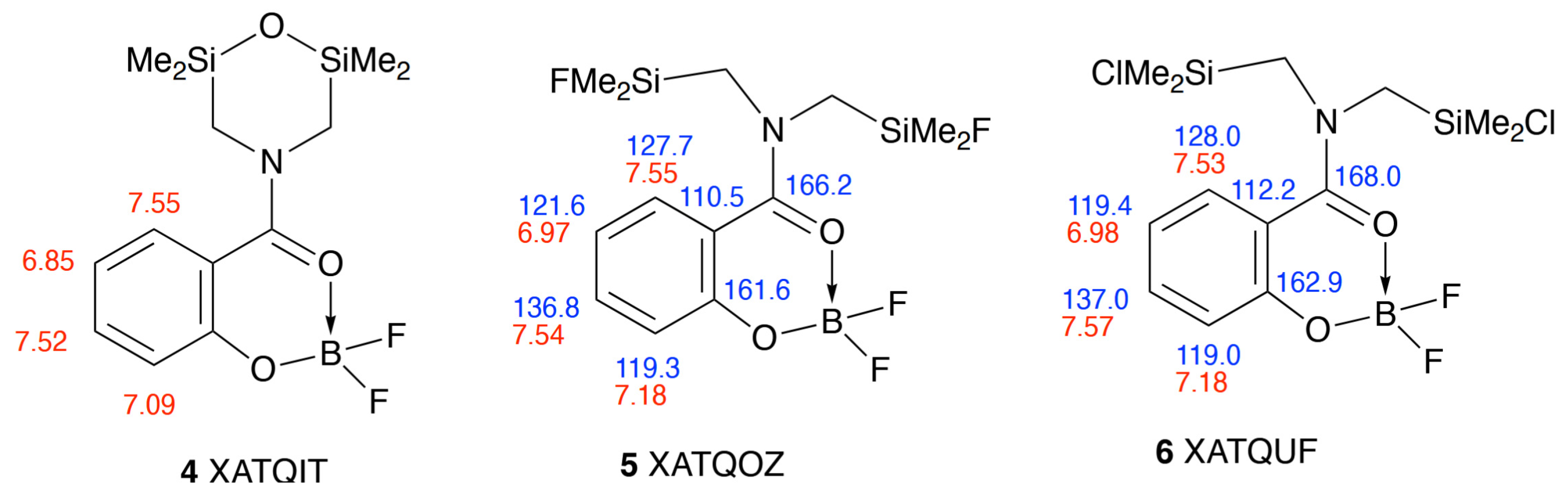
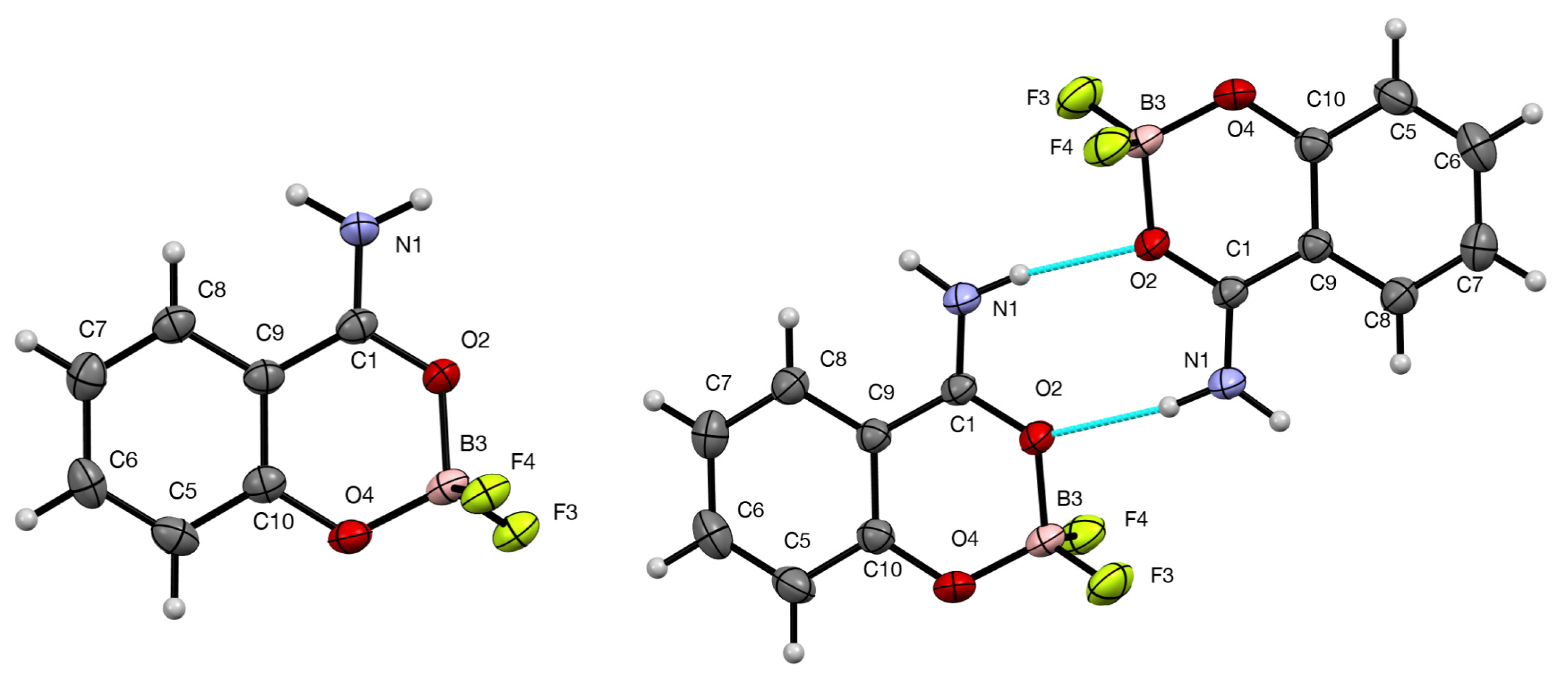
2. Results
| Bond Lengths (Å) | 3 Form A | 3 Form B | 9 |
|---|---|---|---|
| C(1)–N(1) | 1.304(3) | 1.3049(14) | 1.297(3) |
| C(1)–O(2) | 1.298(3) | 1.2949(13) | 1.284(3) |
| O(2)–B(3) | 1.496(3) | 1.5163(15) | 1.505(4) |
| B(3)–O(4) | 1.446(4) | 1.4332(16) | 1.449(4) |
| O(4)–C(10) | 1.348(3) | 1.3444(14) | 1.340(3 |
| C(10)–C(9) | 1.409(4) | 1.4052(15) | 1.395(3) |
| C(9)–C(1) | 1.448(4) | 1.4476(16) | 1.461(4) |
| B(3)–F(3) | 1.383(3) | 1.3641(16) | 1.372(4) |
| B(3)–F(5) | 1.398(4) | 1.3926(16) | 1.375(4) |
| Angles (°) | |||
| C(9)–C(1)–O(2) | 120.7(2) | 120.03(9) | 119.6(2) |
| C(9)–C(1)–N(1) | 122.2(3) | 123.31(10) | 122.8(2) |
| O(2)–C(1)–N(1) | 117.1(3) | 116.66(10) | 117.6(2) |
| C(1)–O(2)–B(3) | 121.5(2) | 121.46(9) | 122.3(2) |
| O(2)–B(3)–O(4) | 112.1(2) | 110.77(9) | 111.5(2) |
| B(3)–O(4)–C(10) | 119.8(2) | 119.04(9) | 118.3(2) |
| O(4)–C(10)–C(9) | 121.0(3) | 121.57(10) | 121.8(2) |
| C(10)–C(9)–C(1) | 117.4(2) | 117.43(10) | 117.2(2) |
| D–H···A | D–H | H···A | D···A | D–H···A | Symmetry Operator |
|---|---|---|---|---|---|
| Form A | |||||
| N(1)–H(1B)···F(4) | 0.93(3) | 1.91(3) | 2.829(3) | 170(3) | 2 − x, 1⁄2 + y, 3⁄2 − z |
| N(1)–H(1A)···F(3) | 0.95(3) | 2.02(3) | 2.919(3) | 159(3) | x, 3⁄2 − y, 1⁄2 − z |
| Form B | |||||
| N(1)–H(1A)···O(2) | 0.929(15) | 2.088(15) | 3.0074(14) | 170.1(13) | 1 − x, −y, 1 − z |
| N(1)–H(1B)···F(4) | 0.910(14) | 2.009(14) | 2.8690(12) | 156.9(14) | 1 + x, y, z |
3. Experimental
3.1. General Experimental Details
3.2. Synthesis of 2-(Difluoroboryloxy)benzamide 3
3.3. X-Ray Structure Determination of 3
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ben-Ishai, D.; Warshawsky, A. The reactions of 2-ethoxy-2,3-dihydro-4H-1,3-benzoxazin-4-one with conjugated dienes. J. Heterocycl. Chem. 1971, 8, 865–866. [Google Scholar] [CrossRef]
- Dey, K.; Gangopadhyay, A.; Biswas, A.K. Synthesis and characterisation of some new fluoroboron complexes with monobasic and dibasic bidentate and dibasic tridentate ligands. Proc. Nat. Acad. Sci. India 1989, 59, 5–15. [Google Scholar]
- Runti, C.; D’Osualdo, V.; Ulian, F. Reazioni fra ortoformiato di etile e composti organici azotati. Nota II. Amidi. Ann. Chim. 1959, 59, 1668–1676. [Google Scholar]
- Korlyukov, A.A.; Lyssenko, K.A.; Antipin, M.Y.; Shipov, A.G.; Zamyshlyaeva, O.A.; Kramarova, E.P.; Negrebetsky, V.V.; Pogozhikh, S.A.; Ovchinnikov, Y.E.; Baukov, Y.I. Synthesis, molecular and crystal structures, and characteristic features of electronic structures of salicylamide-based B, Si-containing chelates. Russ. Chem. Bull. 2004, 53, 1924–1931. [Google Scholar] [CrossRef]
- Judd, K.E.; Mahon, M.F.; Caggiano, L. Efficient synthesis of tetrahydro-β-carbolin-1-one and dihydroisoquinolin-1-one derivatives as versatile intermediates. Synthesis 2009, 2009, 2809–2817. [Google Scholar] [CrossRef]
- Wang, H.; Schrage, B.R.; Takematsu, K.; Ziegler, C.J. Photophysical properties of a boron analogue of coumarin. Phys. Chem. Chem. Phys. 2021, 23, 18855–18862. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Wu, D.-E.; Song, W.-T.; Ge, S.-W.; Sun, B.-W. Positions of amino groups on ammonium salts tunes the conformations of crown ethers: Crystal structures, Hirshfeld surfaces and spectroscopic studies. CrystEngComm 2014, 16, 5319–5330. [Google Scholar] [CrossRef]
- Spilker, A. Ueber neue stickstoffhaltige Abkömmlinge der Salicylsäure. Ber. Dtsch. Chem. Ges. 1889, 22, 2767–2789. [Google Scholar] [CrossRef]
- Rigaku Corporation. CrysAlisPro, v1.171.42.94a, 43.142a and 43.144a; Rigaku Oxford Diffraction: Tokyo, Japan, 2023, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
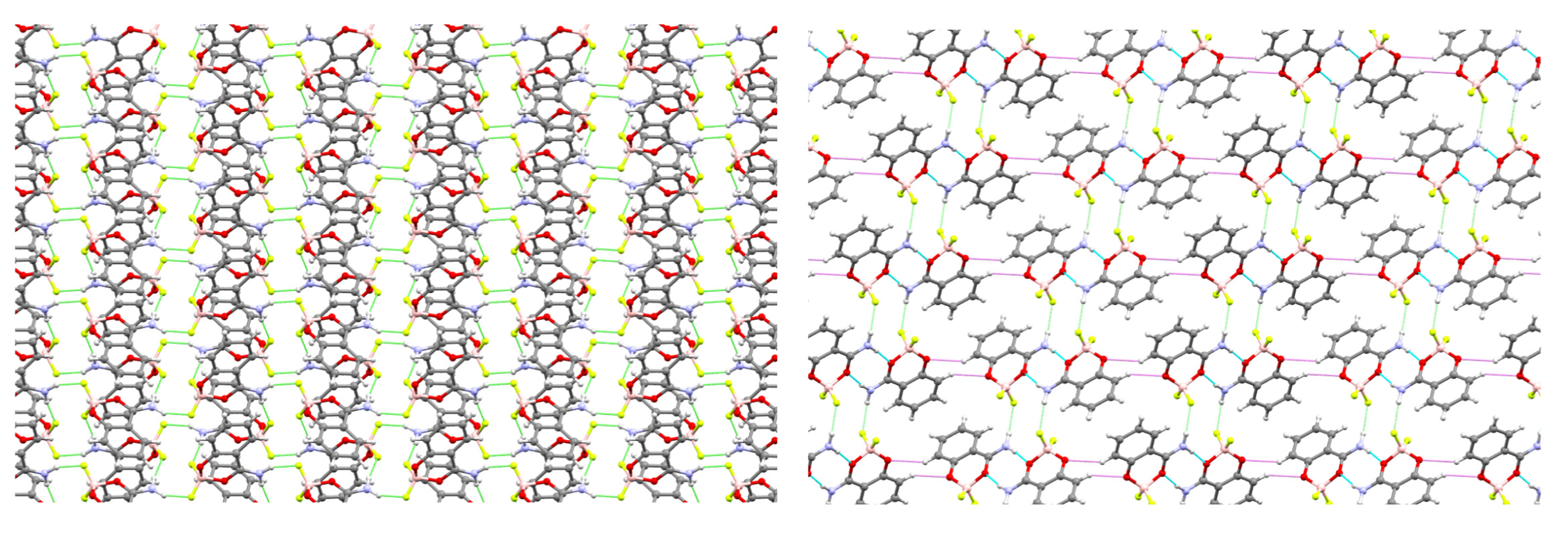
| Solvent | 3JH3–H4 | 3JH4–H5 | 3JH5–H6 | 4JH3–H5 | 4JH4–H6 | 5JH3–H6 |
|---|---|---|---|---|---|---|
| CDCl3 | 8.4 | 7.2 | 8.0 | 0.8 | 1.6 | ~0 |
| CD3OD | 8.4 | 7.2 | 8.0 | 1.2 | 2.0 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Akkache, H.E.M.; Cordes, D.B.; McKay, A.P.; Moreau, D. 2-(Difluoroboryloxy)benzamide. Molbank 2025, 2025, M2093. https://doi.org/10.3390/M2093
Aitken RA, Akkache HEM, Cordes DB, McKay AP, Moreau D. 2-(Difluoroboryloxy)benzamide. Molbank. 2025; 2025(4):M2093. https://doi.org/10.3390/M2093
Chicago/Turabian StyleAitken, R. Alan, Hibet E. M. Akkache, David B. Cordes, Aidan P. McKay, and Dorian Moreau. 2025. "2-(Difluoroboryloxy)benzamide" Molbank 2025, no. 4: M2093. https://doi.org/10.3390/M2093
APA StyleAitken, R. A., Akkache, H. E. M., Cordes, D. B., McKay, A. P., & Moreau, D. (2025). 2-(Difluoroboryloxy)benzamide. Molbank, 2025(4), M2093. https://doi.org/10.3390/M2093








