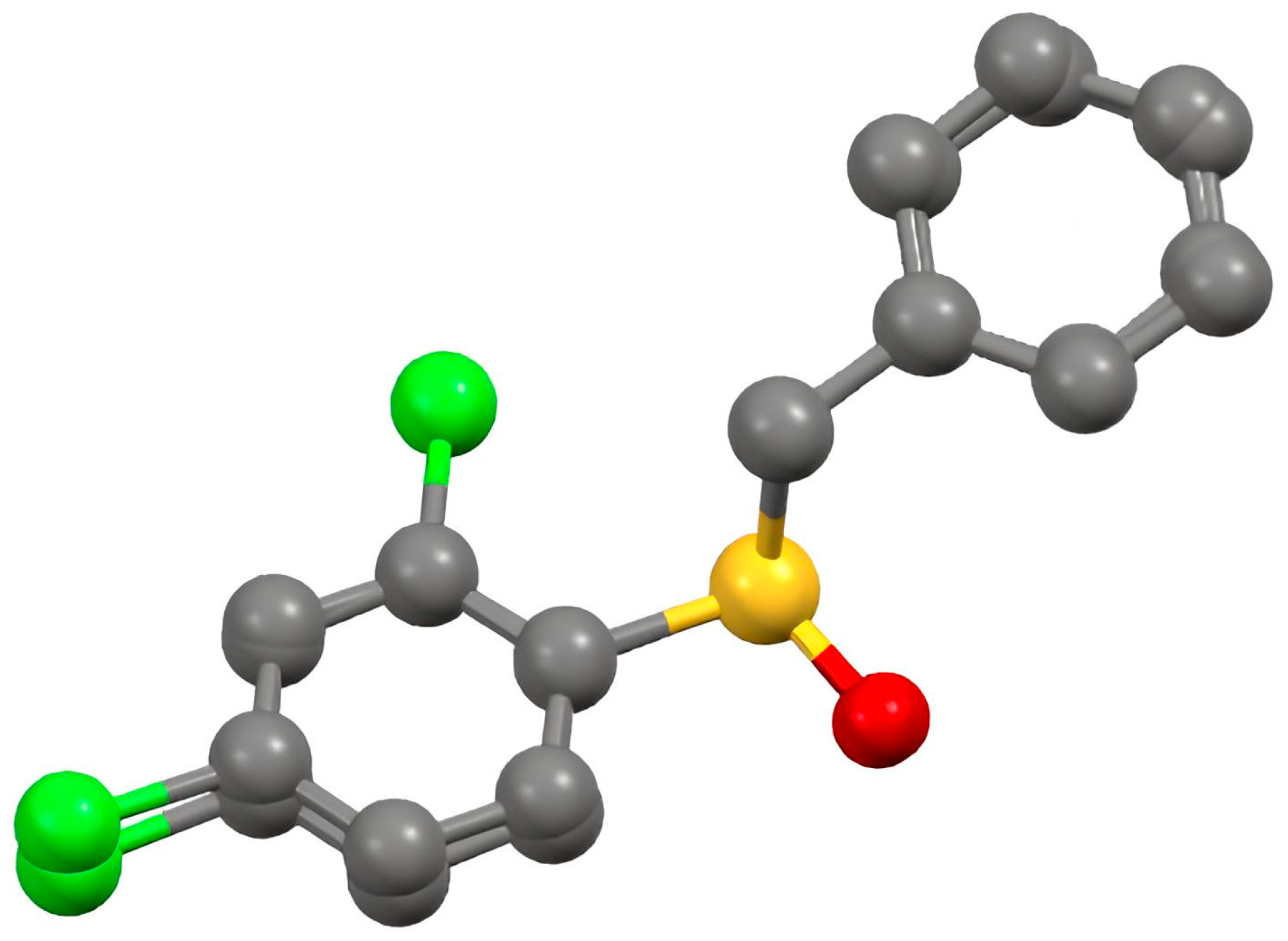
Benzyl 2,4-dichlorophenyl sulfoxide
Abstract
1. Introduction
2. Results
2.1. Synthesis of Racemic and Enantiopure Benzyl 2,4-dichlorophenyl sulfoxide
2.2. Crystal Structure of Racemic Benzyl 2,4-dichlorophenyl sulfoxide
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Empirical formula | C13H10Cl2OS |
| Formula weight | 285.17 |
| Temperature | 296 (2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| Unit cell dimensions | a = 10.0619 (10) Å α = 90°. |
| b = 5.6732 (6) Å β = 99.870 (2)°. | |
| c = 23.360 (3) Å γ = 90°. | |
| Volume | 1313.7 (3) Å3 |
| Z | 4 |
| Density (calculated) | 1.442 Mg/m3 |
| Absorption coefficient | 0.632 mm−1 |
| F (000) | 584 |
| Crystal size | 0.37 × 0.15 × 0.04 mm3 |
| Theta range for data collection | 1.770 to 28.730°. |
| Index ranges | −13 ≤ h ≤ 13, −7 ≤ k ≤ 7, −30 ≤ l ≤ 30 |
| Reflections collected | 10,029 |
| Independent reflections | 3214 [R(int) = 0.0503] |
| Completeness to theta = 25.000° | 100.0 % |
| Absorption correction | Semi-empirical from equivalents |
| Max. and min. transmission | 1 and 0.83 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3214/0/160 |
| Goodness-of-fit on F2 | 1.011 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0573, wR2 = 0.1153 |
| R indices (all data) | R1 = 0.1241, wR2 = 0.1385 |
| Largest diff. peak and hole | 0.287 and −0.236 e.Å−3 |
References
- Wojaczyńska, E.; Wojaczyński, J. Enantioselective Synthesis of Sulfoxides 2000–2009. Chem. Rev. 2010, 110, 4303–4356. [Google Scholar] [CrossRef] [PubMed]
- Wojaczyńska, E.; Wojaczyński, J. Modern Stereoselective Methods of Chiral Sulfinyl Compounds. Chem. Rev. 2020, 120, 4578–4611. [Google Scholar] [CrossRef] [PubMed]
- Wojaczyńska, E.; Wojaczyński, J. Sulfoxides in medicine. Curr. Opin. Chem. Biol. 2023, 76, 102340. [Google Scholar] [CrossRef] [PubMed]
- Surur, A.S.; Schulig, L.; Link, A. Interconnection of sulfides and sulfoxides in medicinal chemistry. Arch. Pharm.—Chem. Life Sci. 2019, 352, e1800248. [Google Scholar] [CrossRef]
- Han, J.; Soloshonok, V.A.; Klika, K.D.; Drabowicz, J.; Wzorek, A. Chiral sulfoxides: Advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C. Organosulfur compounds as chiral building blocks. In Chiral Building Blocks in Asymmetric Synthesis: Synthesis and Application; Wojaczynska, E., Wojaczinski, J., Eds.; Wiley VCH: Weinheim, Germany, 2022; pp. 441–462. ISBN 978-3-527-34946-3. [Google Scholar]
- Wang, C.-R.; Sun, J.-N.; Li, Y.; Li, J.-H. Recent advances in Catalytic Asymmetric Synthesis of Chiral Sulfinyl Compounds. Eur. J. Org. Chem. 2025, 28, e202500378. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective titanium-catalyzed transformations. Coord. Chem. Rev. 2022, 463, 214537. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Cardellicchio, C.; Naso, F. Enantioselective Routes to Sulfoxides Based Upon the Use of Carbanionic Leaving Groups. Eur. J. Org. Chem. 2004, 2004, 1855–1863. [Google Scholar] [CrossRef]
- Naso, F.; Capozzi, M.A.M.; Bottoni, A.; Calvaresi, M.; Bertolasi, V.; Capitelli, F.; Cardellicchio, C. A Combined Theoretical and Experimental Investigation on the Enantioselective Oxidation of Aryl Benzyl Sulfides in the Presence of a Chiral Titanium Catalyst. Chem. Eur. J. 2009, 15, 13417–13426. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Capitelli, F.; Bottoni, A.; Calvaresi, M.; Cardellicchio, C. The Effect of the Fluorine Substitution on the Enantioselective Oxidation of Sulfides Using Chiral Titanium Catalyst. A Combined Computational and Experimental Investigation. ChemCatChem 2013, 5, 210–219. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Capitelli, F.; Cardellicchio, C. Structural Motifs in Enantiopure Halogenated Aryl Benzyl Sulfoxides: Effect of Fluorine Substitution. Cryst. Growth Des. 2014, 14, 5442–5451. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Terraneo, G.; Cavallo, G.; Cardellicchio, C. The search for exceptions in the highly enantioselective titanium catalysed oxidation of aryl benzyl sulfides. Tetrahedron 2015, 71, 4810–4816. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella Febrer, J.F.; Cardellicchio, C. Investigation on the titanium-mediated catalytic enantioselective oxidation of aryl benzyl sulfides containing heterocyclic groups. RSC Adv. 2024, 14, 35105–35113. [Google Scholar] [CrossRef] [PubMed]
- Cardellicchio, C.; Laquintana, V.; Iacobazzi, R.M.; Denora, N.; Scilimati, A.; Perrone, M.G.; Capozzi, M.A.M. Synthesis and Preliminary Screening of the Biological Activity of Sulindac Sulfoximine Derivatives. Appl. Sci. 2023, 13, 12002. [Google Scholar] [CrossRef]
- Capozzi, M.A.M.; Alvarez-Larena, A.; Piniella Febrer, J.F.; Cardellicchio, C. Comparison between the crystal structures of racemic and enantiopure aryl benzyl sulfoxides. RSC Adv. 2025, 15, 37824–37832. [Google Scholar] [CrossRef]
- Fuller, A.F.; Aitken, R.A.; Ryan, B.M.; Slawin, A.M.Z.; Woollins, J.D. The X-Ray Structures of Sulfoxides. J. Chem. Cryst. 2009, 39, 407–415. [Google Scholar] [CrossRef]
- Brondel, N.; Moynihan, E.J.A.; Lehane, K.N.; Eccles, K.S.; Elcoate, C.K.; Coles, S.J.; Lawrence, S.E.; Maguire, A.R. Does intermolecular S=O⋯H-C-S=O hydrogen bonding in sulfoxides and sulfones provide a robust supramolecular synthon in the solid state? CrystEngComm 2010, 12, 2910–2927. [Google Scholar] [CrossRef]
- Thompson, A.L.; White, N.G. Hydrogen atoms in supramolecular chemistry: A structural perspective. Where are they, and why does it matter? Chem. Soc. Rev. 2023, 52, 6254–6269. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 14, 1555–1573. [Google Scholar] [CrossRef]
- Beran, G.J.O. Frontiers of molecular crystal structure prediction for pharmaceuticals and functional organic materials. Chem. Sci. 2023, 14, 13290–13312. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]


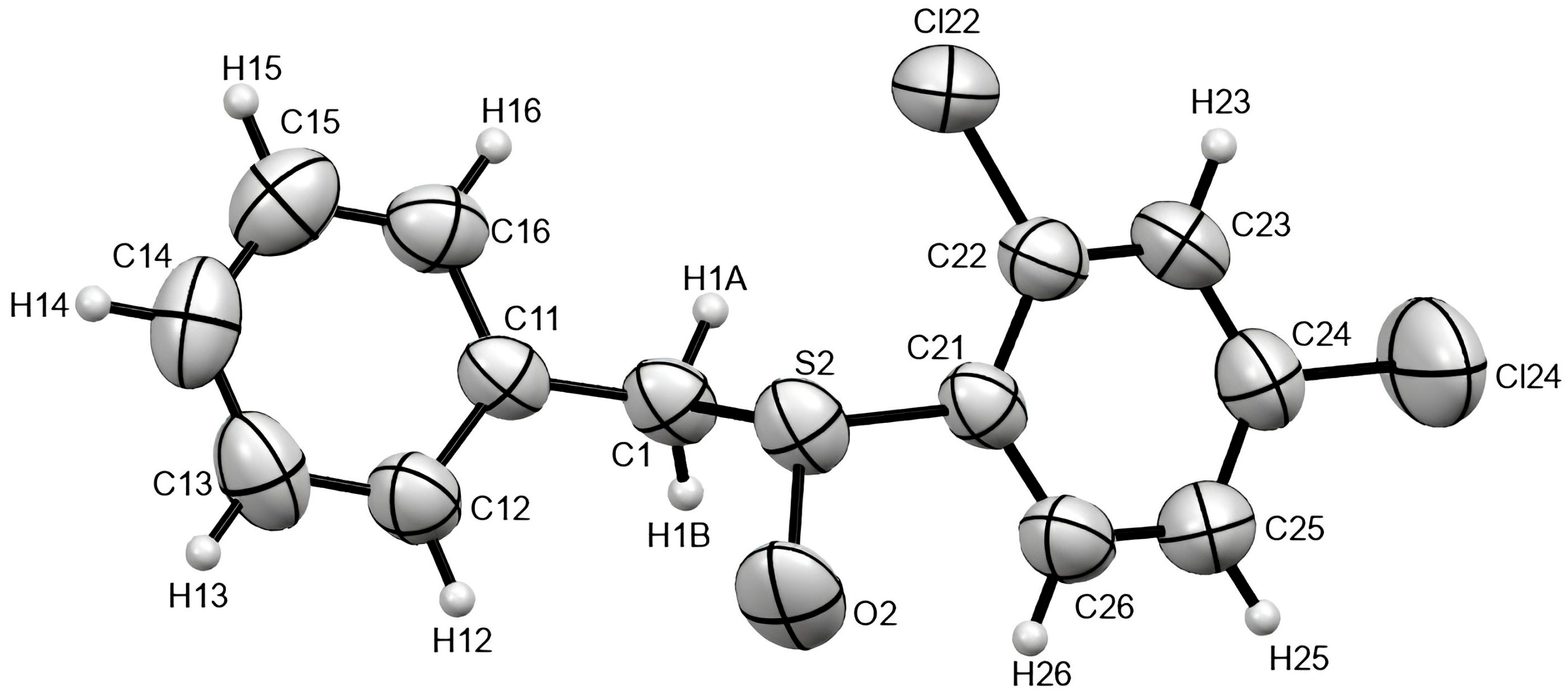
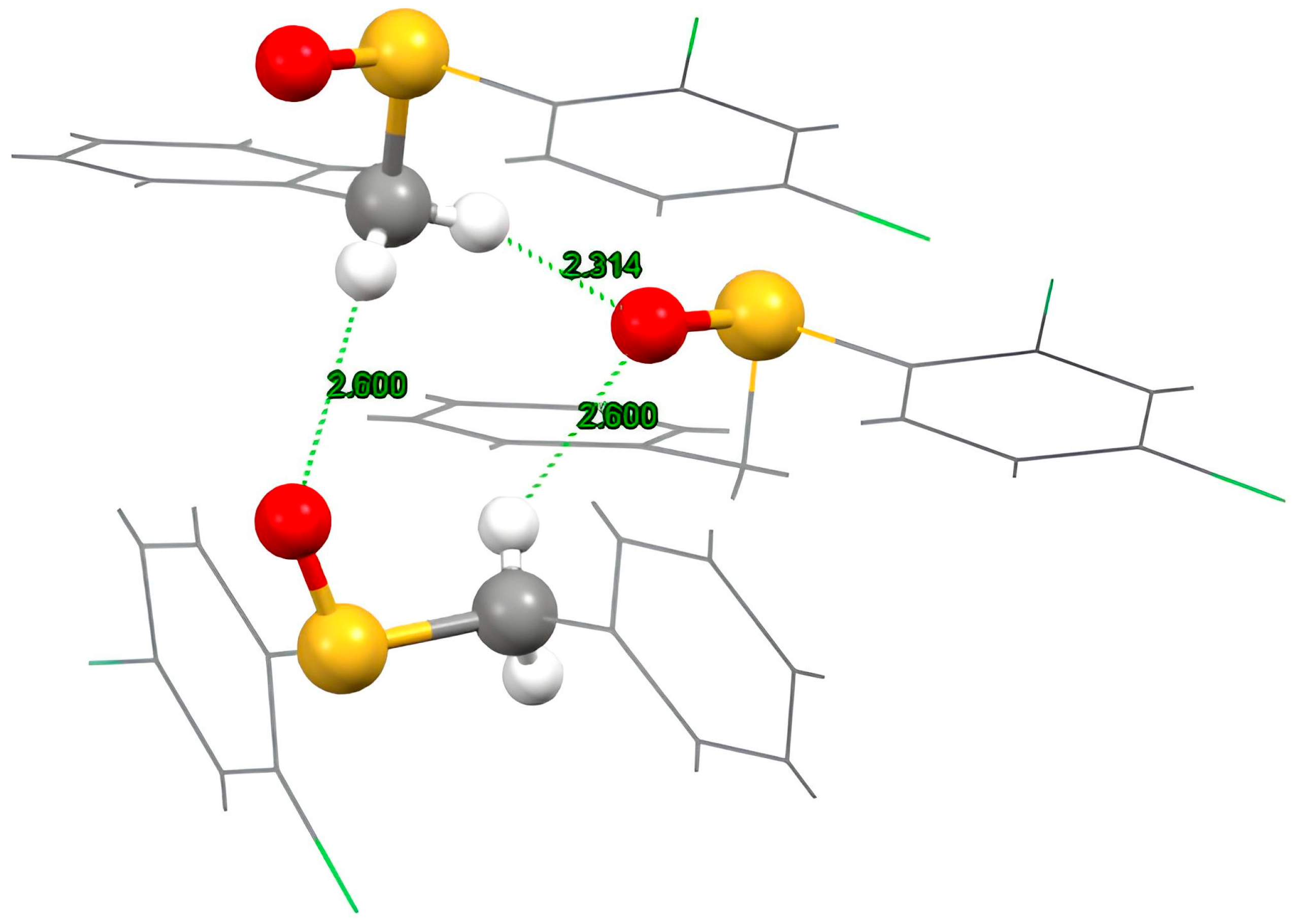
| Angle (°) | H·O (Å) | C·O (Å) | H-C (Å) | |
|---|---|---|---|---|
| C1-H1A-O2 | 160 (2) | 2.31 (3) | 3.251 (3) | 0.98 (3) |
| C1-H1B-O2 | 144 (2) | 2.60 (3) | 3.385 (5) | 0.92 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, M.A.M.; Piniella Febrer, J.F.; Cardellicchio, C. Benzyl 2,4-dichlorophenyl sulfoxide. Molbank 2025, 2025, M2113. https://doi.org/10.3390/M2113
Capozzi MAM, Piniella Febrer JF, Cardellicchio C. Benzyl 2,4-dichlorophenyl sulfoxide. Molbank. 2025; 2025(4):M2113. https://doi.org/10.3390/M2113
Chicago/Turabian StyleCapozzi, Maria Annunziata M., Joan F. Piniella Febrer, and Cosimo Cardellicchio. 2025. "Benzyl 2,4-dichlorophenyl sulfoxide" Molbank 2025, no. 4: M2113. https://doi.org/10.3390/M2113
APA StyleCapozzi, M. A. M., Piniella Febrer, J. F., & Cardellicchio, C. (2025). Benzyl 2,4-dichlorophenyl sulfoxide. Molbank, 2025(4), M2113. https://doi.org/10.3390/M2113







