(4R,4aS,6bR,8aR,12bS,14aS)-2-((E)-2-Bromo-4-chlorobenzylidene)-4,4a,6b,8a,11,11,12b,14a-octamethylicosahydropicen-3(2H)-one
Abstract
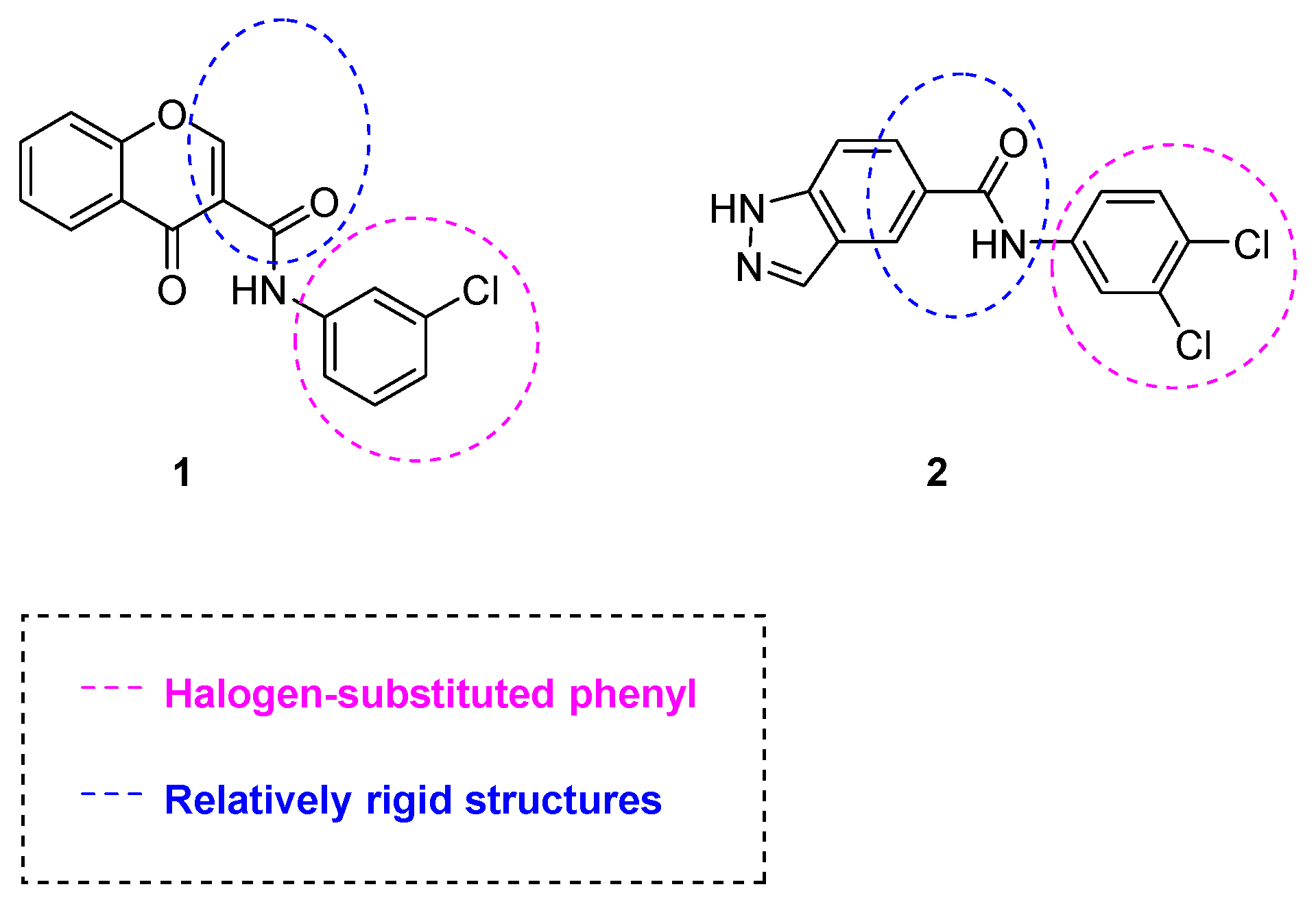
1. Introduction
2. Results and Discussion
Data Analysis
3. Experimental
3.1. General Experimental Conditions
3.2. Synthesis of Friedelin Derivative
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMR | nuclear magnetic resonance |
| HRMS | high-resolution mass spectrometry |
References
- Domingo-Fernández, D.; Gadiya, Y.; Preto, A.J.; Krettler, C.A.; Mubeen, S.; Allen, A.; Healey, D.; Colluru, V. Natural Products Have Increased Rates of Clinical Trial Success throughout the Drug Development Process. J. Nat. Prod. 2024, 87, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Omaveloxolone: First Approval. Drugs 2023, 83, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, A. Ibrexafungerp: First Approval. Drugs 2021, 81, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sassetti, E.; Clausen, M.H.; Laraia, L. Small-Molecule Inhibitors of Reactive Oxygen Species Production. J. Med. Chem. 2021, 64, 5252–5275. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Cagide, F.; Chavarria, D.; Silva, T.; Fernandes, C.; Gaspar, A.; Uriarte, E.; Remião, F.; Alcaro, S.; Ortuso, F.; et al. Discovery of New Chemical Entities for Old Targets: Insights on the Lead Optimization of Chromone-Based Monoamine Oxidase B (MAO-B) Inhibitors. J. Med. Chem. 2016, 59, 5879–5893. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, N.T.; Hinz, S.; Küppers, P.; Gastreich, M.; Müller, C.E. Indazole- and Indole-5-carboxamides: Selective and Reversible Monoamine Oxidase B Inhibitors with Subnanomolar Potency. J. Med. Chem. 2014, 57, 6679–6703. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Ursprung, J.J. The Structures of the Triterpenes Friedelin and Cerin1,2. J. Am. Chem. Soc. 1956, 78, 5041–5051. [Google Scholar] [CrossRef]
- Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Antioxidant, free radical scavenging and liver protective effects of friedelin isolated from Azima tetracantha Lam. leaves. Food Chem. 2013, 139, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.B.; Jeya Ananthi, J.D.; Sathish Kumar, P. Antimicrobial activity of bioactive compounds and leaf extracts in Jatropha tanjorensis. Fitoterapia 2012, 83, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, G.; El Ashry, E.S.H.; Said, M.M.; Aziz, Y.M.A.; Al-Dhfyan, A.; Al-Majid, A.M.; Barakat, A. Regio- and stereoselective synthesis of new spirooxindoles via 1,3-dipolar cycloaddition reaction: Anticancer and molecular docking studies. J. Photochem. Photobiol. B 2018, 180, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.; Haddad, N. Steric effects in intramolecular [2+2] photocycloaddition of C C double bonds to cyclohexenones. Tetrahedron 1993, 49, 947–964. [Google Scholar] [CrossRef]
- Piacenza, L.P.L.; Pegel, K.H.; Laing, M.; Waight, E.S.; Weeks, C.M.; Gorst-Allman, C.P. A new atisane diterpene: Ent-16α-hydroxyatis-13-en-3-one from Androstachys johnsonii prain. J. Chem. Soc. Perkin Trans. 1985, 703–709. [Google Scholar] [CrossRef]
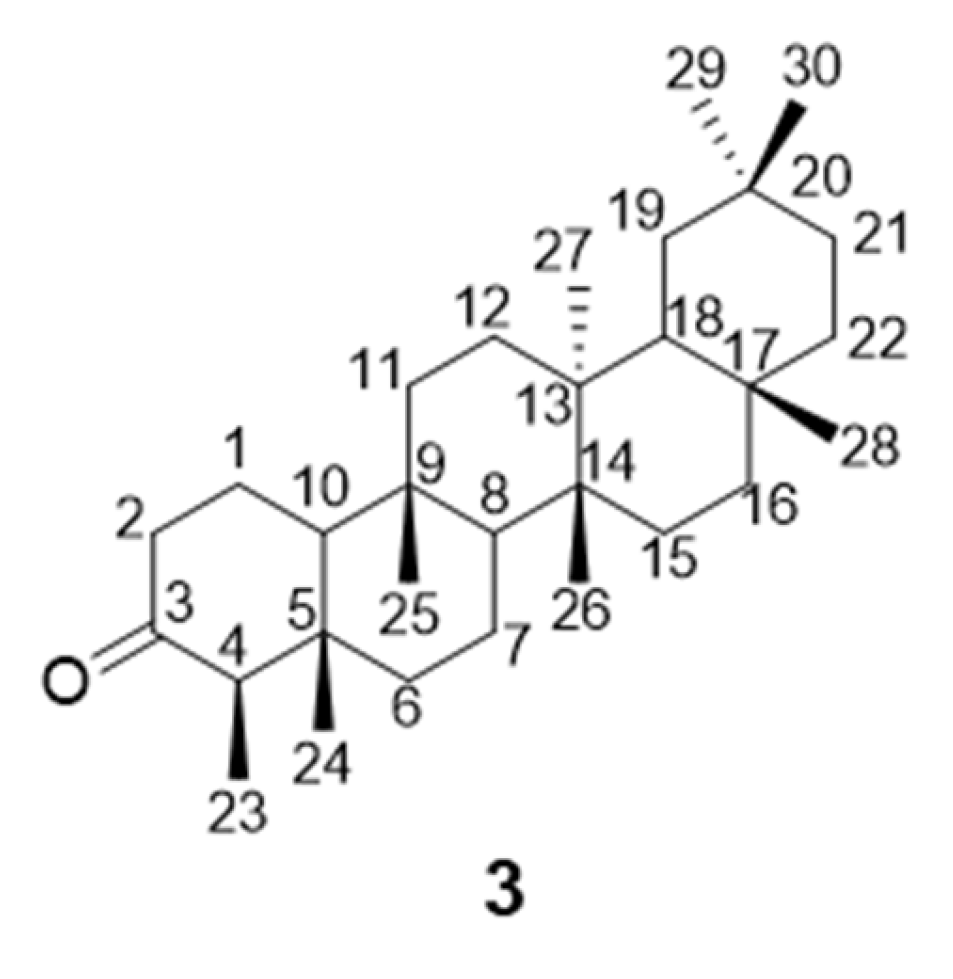


| No. | 13C | 1H | No. | 13C | 1H | No. | 13C | 1H |
|---|---|---|---|---|---|---|---|---|
| 1 | 25.4 | 2.74 (m), 2.40 (m) | 14 | 38.3 | - | 27 | 18.6 | 0.99 (s) |
| 2 | 139.6 | - | 15 | 32.3 | 1.47 (m), 1.19 (m) | 28 | 32.0 | 1.17 (s) |
| 3 | 204.3 | - | 16 | 35.9 | 1.52 (m), 1.36 (m) | 29 | 34.8 | 0.93 (s) |
| 4 | 58.9 | 2.28 (m) | 17 | 30.0 | - | 30 | 31.9 | 0.98 (s) |
| 5 | 39.5 | - | 18 | 42.7 | 1.53 (m) | 1′ | 134.3 | - |
| 6 | 41.3 | 1.81 (m), 1.27 (m) | 19 | 35.0 | 1.33 (m), 1.14 (m) | 2′ | 125.4 | - |
| 7 | 18.1 | 1.49 (m), 1.41 (m) | 20 | 28.1 | - | 3′ | 131.1 | 7.63 (d, 2.1) |
| 8 | 53.0 | 1.38 (m) | 21 | 32.8 | 1.45 (m), 1.25 (m) | 4′ | 134.7 | - |
| 9 | 37.4 | - | 22 | 39.1 | 1.50 (m), 0.91 (m) | 5′ | 127.1 | 7.30 (dd, 8.3, 2.1) |
| 10 | 57.2 | 1.56 (m) | 23 | 7.6 | 1.05 (d) | 6′ | 131.0 | 7.21 (d, 8.3) |
| 11 | 35.2 | 1.35 (m) 1.15 (m) | 24 | 14.8 | 0.85 (s) | 7′ | 132.6 | 7.17 (m) |
| 12 | 30.1 | 1.29 (m) | 25 | 17.3 | 0.92 (s) | |||
| 13 | 39.6 | - | 26 | 20.2 | 1.01 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, K.; Yu, J.; Chen, Y.; Chen, J.; Zhao, Q.; Hao, X.; Xu, J.; Ding, X. (4R,4aS,6bR,8aR,12bS,14aS)-2-((E)-2-Bromo-4-chlorobenzylidene)-4,4a,6b,8a,11,11,12b,14a-octamethylicosahydropicen-3(2H)-one. Molbank 2025, 2025, M2110. https://doi.org/10.3390/M2110
Guan K, Yu J, Chen Y, Chen J, Zhao Q, Hao X, Xu J, Ding X. (4R,4aS,6bR,8aR,12bS,14aS)-2-((E)-2-Bromo-4-chlorobenzylidene)-4,4a,6b,8a,11,11,12b,14a-octamethylicosahydropicen-3(2H)-one. Molbank. 2025; 2025(4):M2110. https://doi.org/10.3390/M2110
Chicago/Turabian StyleGuan, Kaichen, Jinzheng Yu, Yangzhonghui Chen, Jianqin Chen, Qian Zhao, Xiaojiang Hao, Juan Xu, and Xiao Ding. 2025. "(4R,4aS,6bR,8aR,12bS,14aS)-2-((E)-2-Bromo-4-chlorobenzylidene)-4,4a,6b,8a,11,11,12b,14a-octamethylicosahydropicen-3(2H)-one" Molbank 2025, no. 4: M2110. https://doi.org/10.3390/M2110
APA StyleGuan, K., Yu, J., Chen, Y., Chen, J., Zhao, Q., Hao, X., Xu, J., & Ding, X. (2025). (4R,4aS,6bR,8aR,12bS,14aS)-2-((E)-2-Bromo-4-chlorobenzylidene)-4,4a,6b,8a,11,11,12b,14a-octamethylicosahydropicen-3(2H)-one. Molbank, 2025(4), M2110. https://doi.org/10.3390/M2110






