Synthesis of 3,5-Diamino-Substituted Dithieno[3,2-b:2′,3′-d]thiophene Derivatives
Abstract
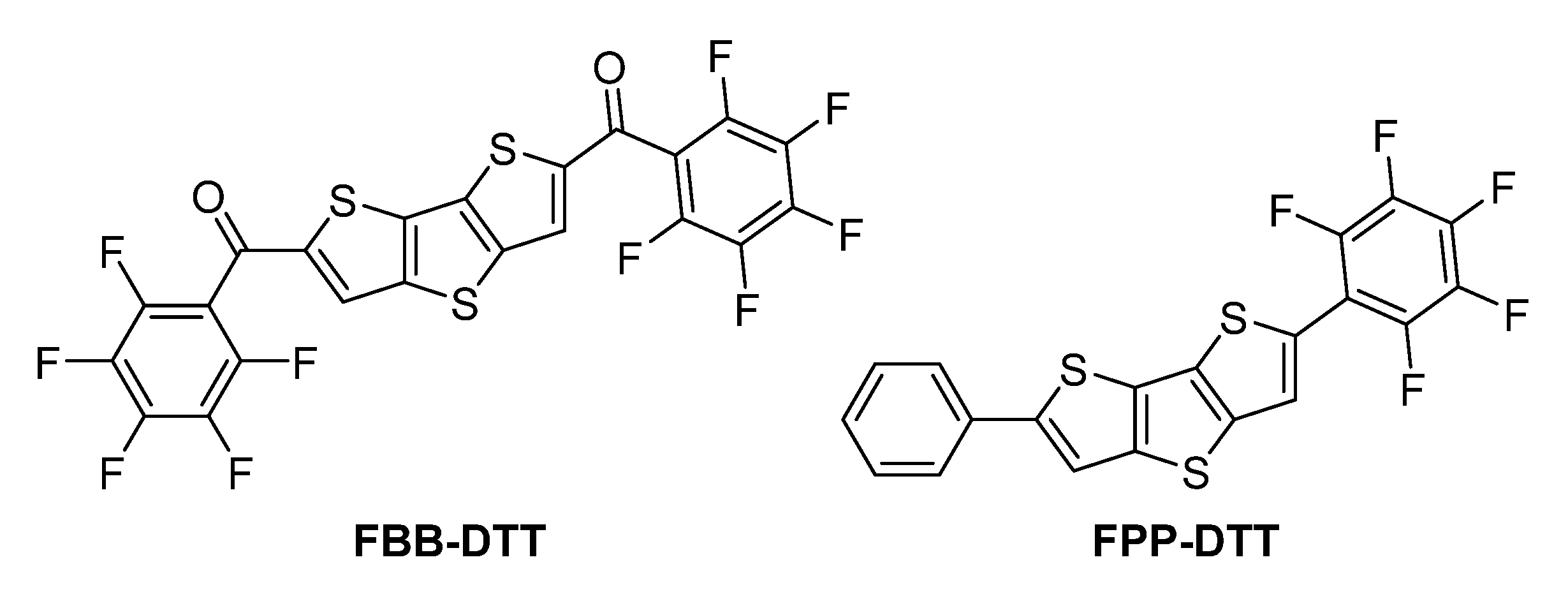
1. Introduction
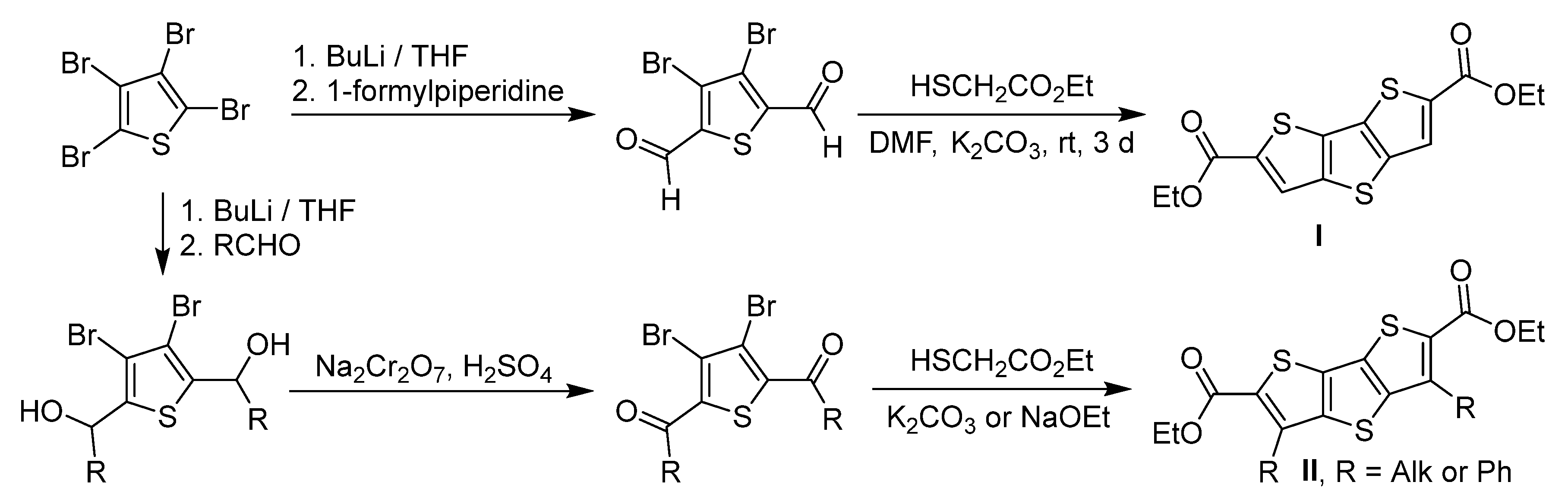
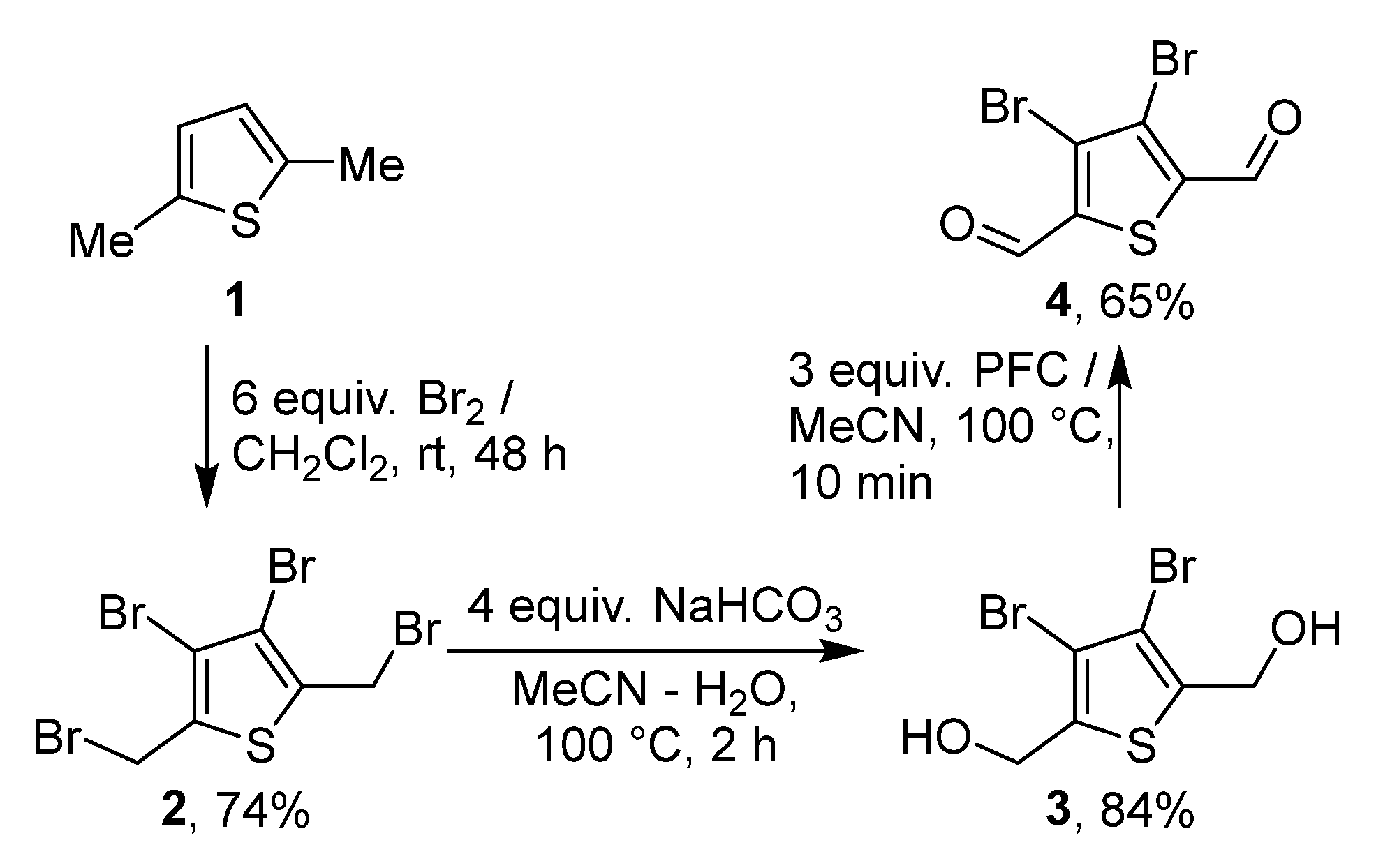
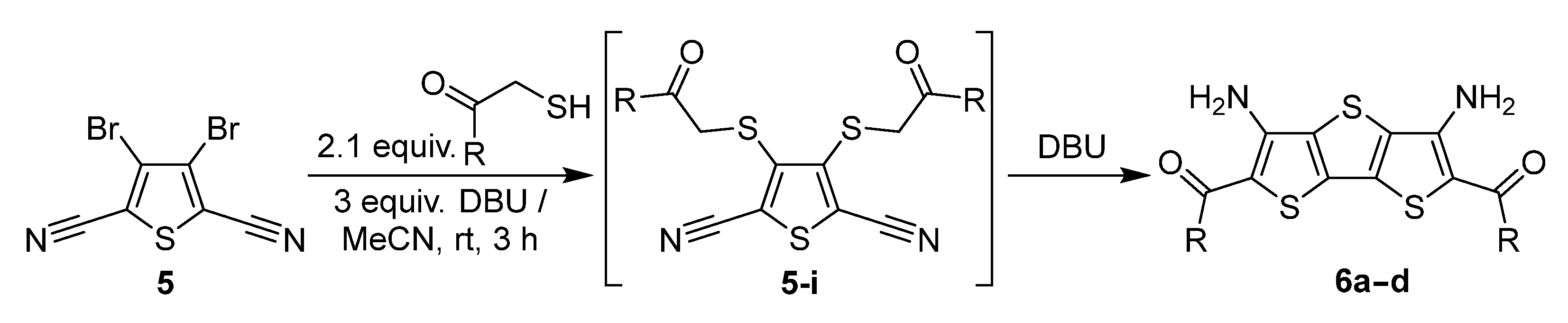
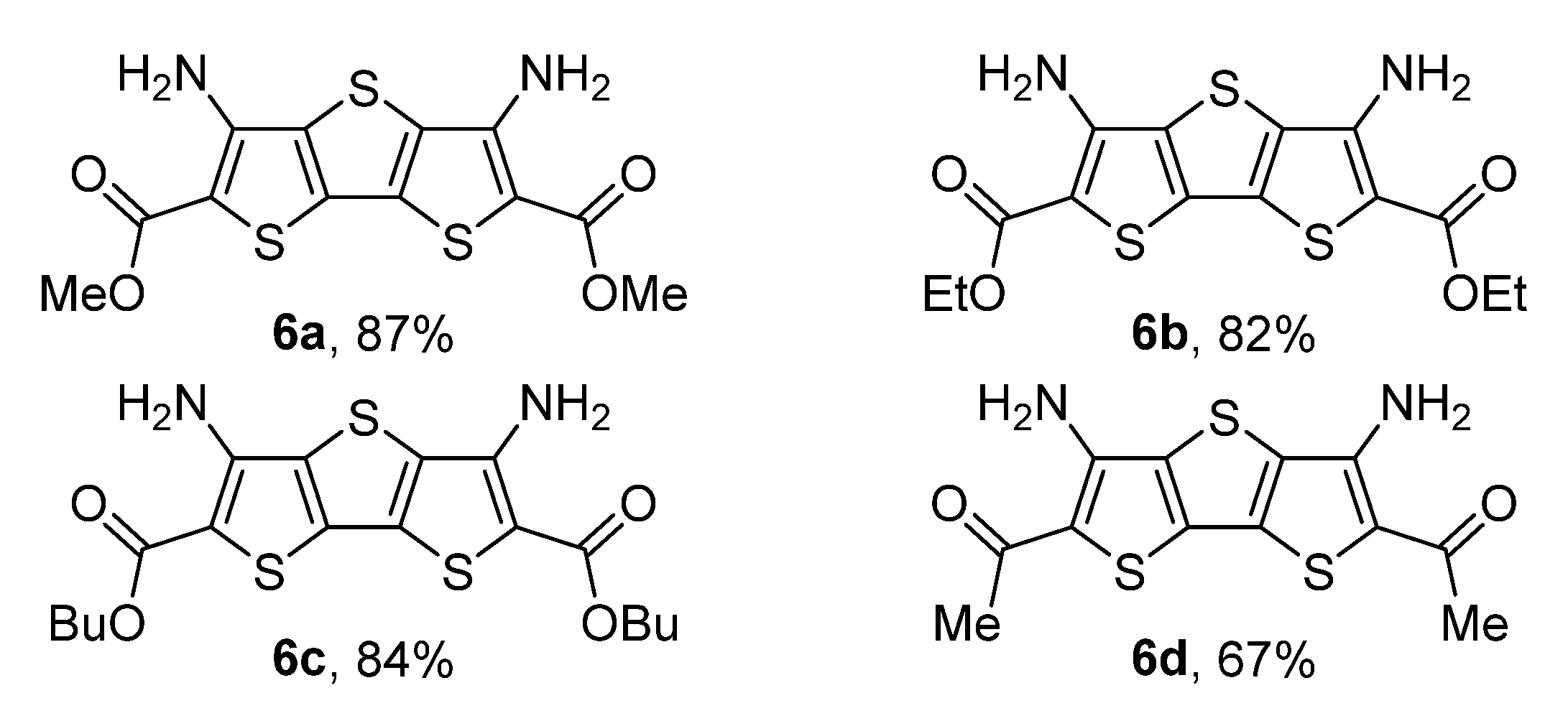
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Thiophene-Based Organic Semiconductors. In Topics in Current Chemistry; Springer: Cham, Switzerland, 2019; Volume 375, pp. 79–123. ISBN 978-3-030-25598-5. [Google Scholar]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-conjugated systems in field-effect transistors: A material odyssey of organic electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef]
- Cinar, M.E.; Ozturk, T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. [Google Scholar] [CrossRef]
- Larik, F.A.; Faisal, M.; Saeed, A.; Abbas, Q.; Kazi, M.A.; Abbas, N.; Thebo, A.A.; Khan, D.M.; Channar, P.A. Thiophene-based molecular and polymeric semiconductors for organic field effect transistors and organic thin film transistors. J. Mater. Sci. Mater. Electron. 2018, 29, 17975–18010. [Google Scholar] [CrossRef]
- Vegiraju, S.; Luo, X.L.; Li, L.H.; Afraj, S.N.; Lee, C.; Zheng, D.; Hsieh, H.C.; Lin, C.C.; Hong, S.H.; Tsai, H.C.; et al. Solution Processable Pseudo n-Thienoacenes via Intramolecular S···S Lock for High Performance Organic Field Effect Transistors. Chem. Mater. 2020, 32, 1422–1429. [Google Scholar] [CrossRef]
- Borshchev, O.V.; Skorotetcky, M.S.; Trukhanov, V.A.; Fedorenko, R.S.; Surin, N.M.; Svidchenko, E.A.; Sosorev, A.Y.; Kazantsev, M.S.; Paraschuk, D.Y.; Ponomarenko, S.A. Synthesis, characterization and organic field-effect transistors applications of novel tetrathienoacene derivatives. Dye. Pigment. 2021, 185, 108911. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, M.; Sun, S.; Shi, Y.; Ma, Z.; Sun, Z.; Xue, S. Synthesis and photovoltaic properties of organic sensitizers containing electron-deficient and electron-rich fused thiophene for dye-sensitized solar cells. Tetrahedron 2012, 68, 5375–5385. [Google Scholar] [CrossRef]
- Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; Yau, S.; Kim, C.; Lee, W.-H.; Chen, M.-C.; Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; et al. Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells. Polymers 2014, 6, 2645–2669. [Google Scholar] [CrossRef]
- Eom, Y.K.; Kang, S.H.; Choi, I.T.; Kim, E.; Kim, J.; Ju, M.J.; Kim, H.K. New thieno[3,2-b][1]benzothiophene-based organic sensitizers containing π-extended thiophene spacers for efficient dye-sensitized solar cells. RSC Adv. 2015, 5, 80859–80870. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Sun, X.; Wang, H.; Hu, H.; Wang, K.; Xiao, M. Small molecule donor third component incorporating thieno[3,2-b]thiophene unit enables 19.18% efficiency ternary organic solar cells with improved operational stability. Nano Energy 2024, 125, 109542. [Google Scholar] [CrossRef]
- Palamà, I.; Di Maria, F.; Viola, I.; Fabiano, E.; Gigli, G.; Bettini, C.; Barbarella, G. Live-Cell-Permeant Thiophene Fluorophores and Cell-Mediated Formation of Fluorescent Fibrils. J. Am. Chem. Soc. 2011, 133, 17777–17785. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Nakano, M.; Kang, M.J.; Miyazaki, E.; Osaka, I. Thienannulation: Efficient Synthesis of π-Extended Thienoacenes Applicable to Organic Semiconductors. Eur. J. Org. Chem. 2013, 2013, 217–227. [Google Scholar] [CrossRef]
- Niebel, C.; Kim, Y.; Ruzié, C.; Karpinska, J.; Chattopadhyay, B.; Schweicher, G.; Richard, A.; Lemaur, V.; Olivier, Y.; Cornil, J.; et al. Thienoacene dimers based on the thieno[3,2-b]thiophene moiety: Synthesis, characterization and electronic properties. J. Mater. Chem. C 2014, 3, 674–685. [Google Scholar] [CrossRef]
- Zheng, T.; Cai, Z.; Ho-Wu, R.; Yau, S.H.; Shaparov, V.; Goodson, T.; Yu, L. Synthesis of Ladder-Type Thienoacenes and Their Electronic and Optical Properties. J. Am. Chem. Soc. 2016, 138, 868–875. [Google Scholar] [CrossRef]
- Ali, R.; Siddiqui, R. Dithieno[3,2-b:2′,3′-d]thiophene (DTT): An emerging heterocyclic building block for future organic electronic materials & functional supramolecular chemistry. RSC Adv. 2022, 12, 36073–36102. [Google Scholar] [CrossRef] [PubMed]
- Strakova, K.; Assies, L.; Goujon, A.; Piazzolla, F.; Humeniuk, H.V.; Matile, S. Dithienothiophenes at Work: Access to Mechanosensitive Fluorescent Probes, Chalcogen-Bonding Catalysis, and Beyond. Chem. Rev. 2019, 119, 10977–11005. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chiang, Y.J.; Kim, C.; Guo, Y.J.; Chen, S.Y.; Liang, Y.J.; Huang, Y.W.; Hu, T.S.; Lee, G.H.; Facchetti, A.; et al. One-pot [1+1+1] synthesis of dithieno[2,3-b:3′,2′-d]thiophene (DTT) and their functionalized derivatives for organic thin-film transistors. Chem. Commun. 2009, 1846–1848. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, T.M.; Oh, H.S.; Kim, J.J.; Hong, J.I. Vacuum processable donor material based on dithieno[3,2-b:2′,3′-d]thiophene and pyrene for efficient organic solar cells. RSC Adv. 2014, 4, 24453–24457. [Google Scholar] [CrossRef]
- Ho, D.; Jeon, M.; Kim, H.; Gidron, O.; Kim, C.; Seo, S.Y. Solution-processable dithieno[3,2-b:2′,3′-d]thiophene derivatives for organic thin-film transistors and complementary-like inverters. Org. Electron. 2018, 52, 356–363. [Google Scholar] [CrossRef]
- Park, J.-H.; Kim, U.-Y.; Kim, B.-M.; Kim, W.-H.; Roh, D.-H.; Kim, J.S.; Kwon, T.-H. Molecular Design Strategy toward Robust Organic Dyes in Thin-Film Photoanodes. ACS Appl. Energy Mater. 2019, 2, 4674–4682. [Google Scholar] [CrossRef]
- Frey, J.; Bond, A.D.; Holmes, A.B. Improved synthesis of dithieno[3,2-b:2’,3’-d]thiophene (DTT) and derivatives for cross coupling. Chem. Commun. 2002, 2, 2424–2425. [Google Scholar] [CrossRef]
- He, M.; Zhang, F. Synthesis and structure of alkyl-substituted fused thiophenes containing up to seven rings. J. Org. Chem. 2007, 72, 442–451. [Google Scholar] [CrossRef]
- Benz, S.; Macchione, M.; Verolet, Q.; Mareda, J.; Sakai, N.; Matile, S. Anion Transport with Chalcogen Bonds. J. Am. Chem. Soc. 2016, 138, 9093–9096. [Google Scholar] [CrossRef]
- Macchione, M.; Tsemperouli, M.; Goujon, A.; Mallia, A.R.; Sakai, N.; Sugihara, K.; Matile, S. Mechanosensitive Oligodithienothiophenes: Transmembrane Anion Transport Along Chalcogen-Bonding Cascades. Helv. Chim. Acta 2018, 101, e1800014. [Google Scholar] [CrossRef]
- Strakova, K.; Poblador-Bahamonde, A.I.; Sakai, N.; Matile, S.; Poblador-Bahamonde, A.I.; Sakai, N.; Matile, S.; Poblador-Bahamonde, A.I.; Sakai, N.; Matile, S. Fluorescent Flipper Probes: Comprehensive Twist Coverage. Chem. A Eur. J. 2019, 25, 14935–14942. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Burrezo, P.M.; Lee, S.; Zhang, W.; Zheng, B.; Dai, G.; Chang, J.; López Navarrete, J.T.; Huang, K.W.; Kim, D.; et al. Antiaromatic bisindeno-[n]thienoacenes with small singlet biradical characters: Syntheses, structures and chain length dependent physical properties. Chem. Sci. 2014, 5, 4490–4503. [Google Scholar] [CrossRef]
- Demina, N.S.; Bayankina, P.E.; Irgashev, R.A.; Kazin, N.A.; Rusinov, G.L. An Effective Route to Dithieno[3,2-b:2′,3′-d]thiophene-Based Hexaheteroacenes. Synlett 2021, 32, 1009–1013. [Google Scholar] [CrossRef]
- Deng, H.; Hu, J.; Hu, H.; He, M.; Fang, Y. Thieno[3,2-b]thiophene-2-carboxylic acid derivatives as GPR35 agonists. Bioorg. Med. Chem. Lett. 2012, 22, 4148–4152. [Google Scholar] [CrossRef]
- Liu, Z.; Yasseri, A.A.; Loewe, R.S.; Lysenko, A.B.; Malinovskii, V.L.; Zhao, Q.; Surthi, S.; Li, Q.; Misra, V.; Lindsey, J.S.; et al. Synthesis of Porphyrins Bearing Hydrocarbon Tethers and Facile Covalent Attachment to Si(100). J. Org. Chem. 2004, 69, 5568–5577. [Google Scholar] [CrossRef]
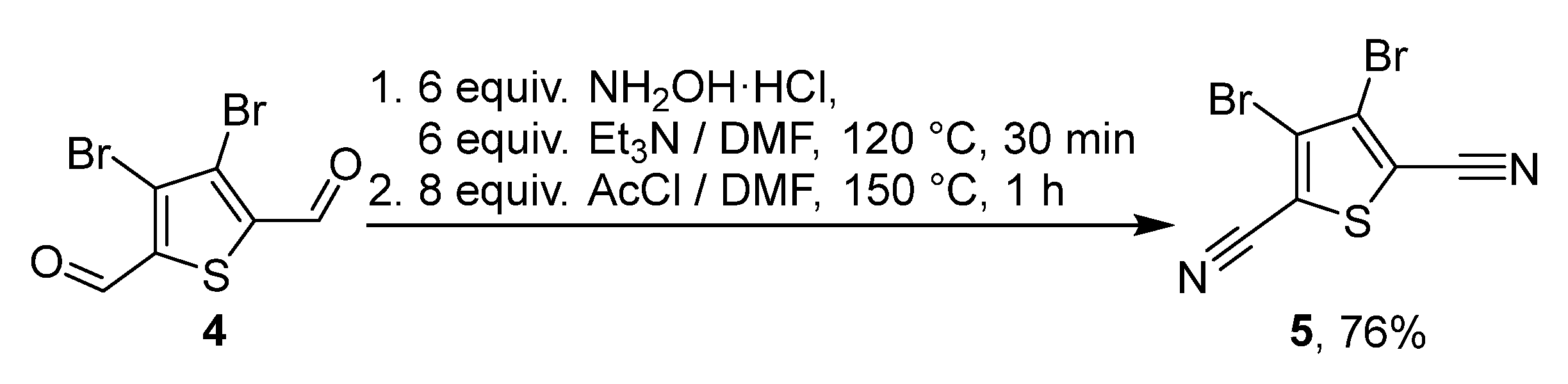
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irgashev, R.A.; Kazin, N.A. Synthesis of 3,5-Diamino-Substituted Dithieno[3,2-b:2′,3′-d]thiophene Derivatives. Molbank 2025, 2025, M2109. https://doi.org/10.3390/M2109
Irgashev RA, Kazin NA. Synthesis of 3,5-Diamino-Substituted Dithieno[3,2-b:2′,3′-d]thiophene Derivatives. Molbank. 2025; 2025(4):M2109. https://doi.org/10.3390/M2109
Chicago/Turabian StyleIrgashev, Roman A., and Nikita A. Kazin. 2025. "Synthesis of 3,5-Diamino-Substituted Dithieno[3,2-b:2′,3′-d]thiophene Derivatives" Molbank 2025, no. 4: M2109. https://doi.org/10.3390/M2109
APA StyleIrgashev, R. A., & Kazin, N. A. (2025). Synthesis of 3,5-Diamino-Substituted Dithieno[3,2-b:2′,3′-d]thiophene Derivatives. Molbank, 2025(4), M2109. https://doi.org/10.3390/M2109




