Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate
Abstract
1. Introduction
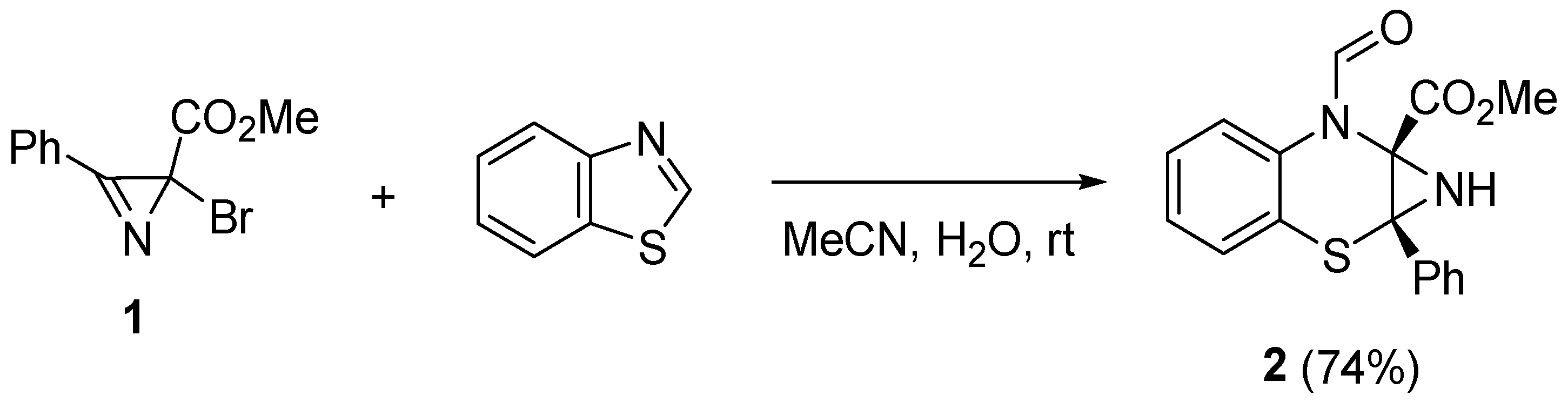
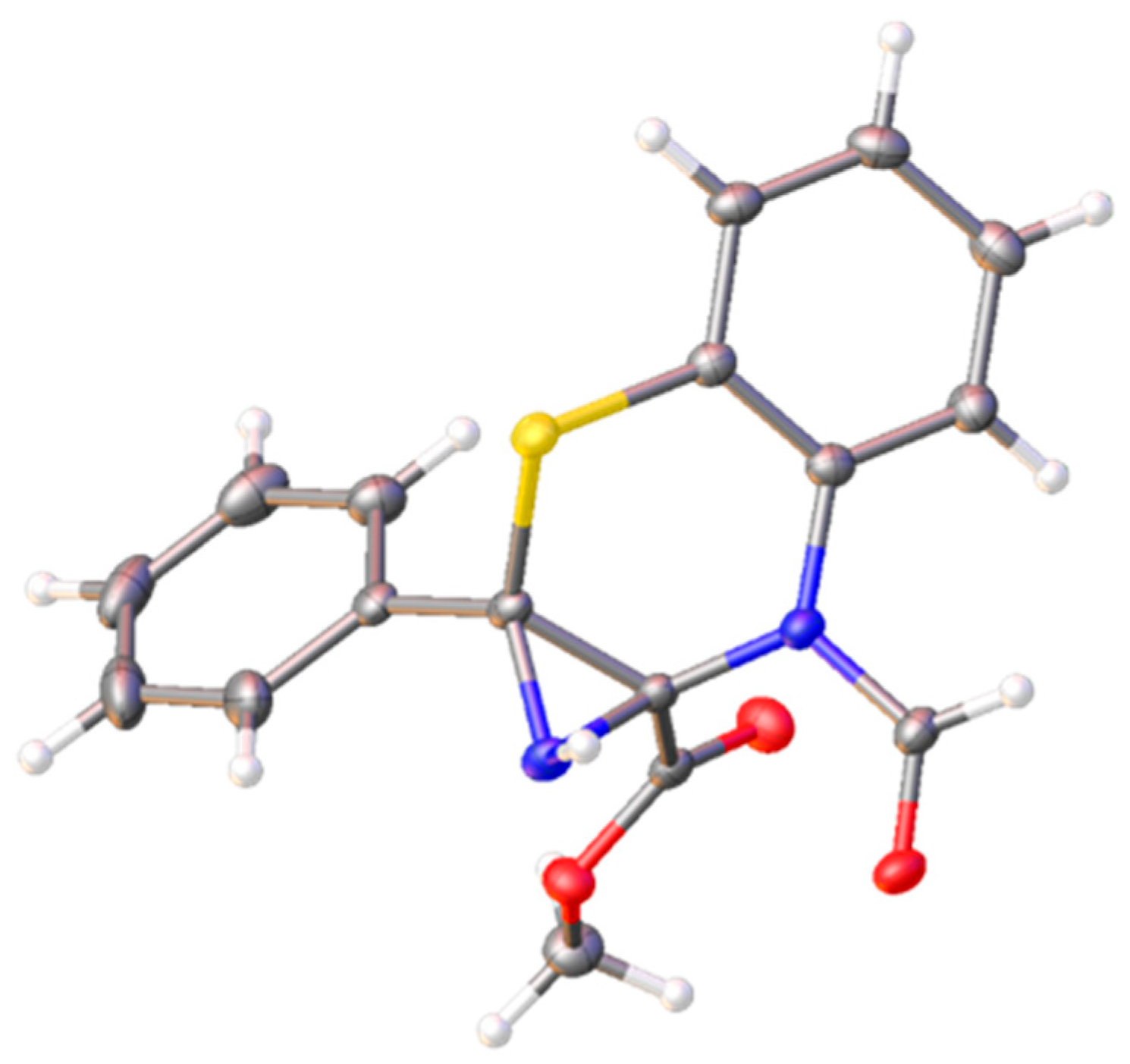
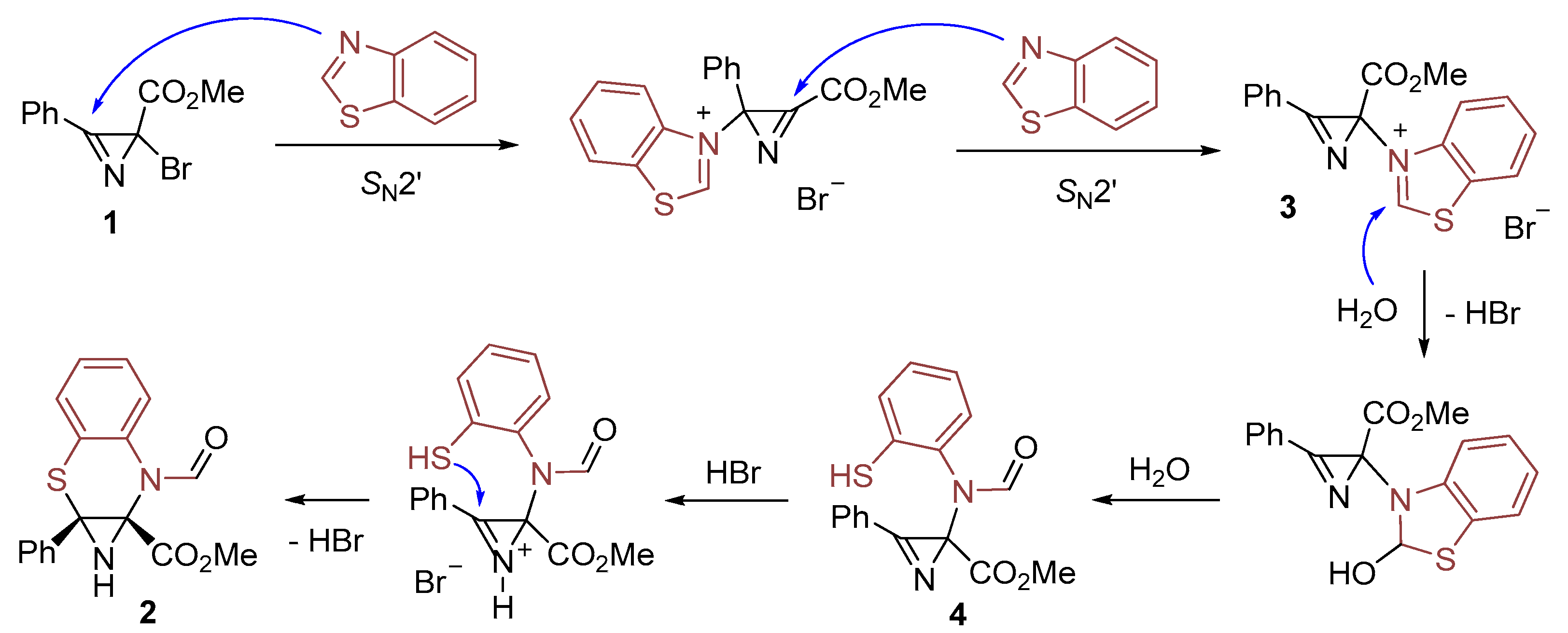
2. Results and Discussion
3. Materials and Methods
3.1. General Instrumentation
3.2. Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.R.; Jain, M.; Rathore, R.S.; Gupta, A. Synthetic and spectral investigation of fluorinated phenothiazines and 4H-l,4-benzothiazines as potent anticancer agents. J. Fluorine Chem. 1993, 62, 191–200. [Google Scholar] [CrossRef]
- Rathore, B.S.; Kumar, M. Synthesis of 7-chloro-5-trifluoromethyl/7-fluoro/7-trifluoromethyl-4H-1,4-benzothiazines as antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 5678–5682. [Google Scholar] [CrossRef]
- Macchiarulo, A.; Costantino, G.; Fringuelli, D.; Vecchiarelli, A.; Schiaffella, F.; Fringuelli, R. 1,4-Benzothiazine and 1,4-benzoxazine imidazole derivatives with antifungal activity: A docking study. Bioorg. Med. Chem. 2002, 10, 3415–3423. [Google Scholar] [CrossRef]
- Vicente, J.D.; Hendricks, R.T.; Smith, D.B.; Fell, J.B.; Fischer, J.; Spencer, S.R.; Stengel, P.J.; Mohr, P.; Robinson, J.E.; Blake, J.F.; et al. Non-nucleoside inhibitors of HCV polymerase NS5B. Part 2: Synthesis and structure-activity relationships of benzothiazine-substituted quinolinediones. Bioorg. Med. Chem. Lett. 2009, 19, 3642–3646. [Google Scholar] [CrossRef] [PubMed]
- Campiani, G.; Garofalo, A.; Fiorini, I.; Botta, M.; Nacci, V.; Tafi, A.; Chiarini, A.; Budriesi, R.; Bruni, G.; Romeo, M.R. Pyrrolo [2,1-c][1,4]benzothiazines synthesis, structure-activity relationships, molecular modeling studies, and cardiovascular activity. J. Med. Chem. 1995, 38, 4393–4410. [Google Scholar] [CrossRef]
- Turk, C.F.; Krapcho, J. 4-(3-(Dimethylamin)propyl-3,4-dihydro-2(1-hydroxyethyl)3-phenyl-2H-1,4-benzothiazine and related compounds: A new class of anti-inflammatory agents. J. Med. Chem. 1973, 16, 776–779. [Google Scholar] [CrossRef]
- Cecchetti, V.; Calderone, V.; Tabarrini, O.; Sabatini, S.; Filipponi, E.; Testai, L.; Spogli, R.; Martinotti, E.; Fravolini, A. Highly potent 1,4-benzothiazine derivatives as KATP-channel openers. J. Med. Chem. 2003, 46, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, K.; Mihara, M.; Takagi, N.; Kawamura, A.; Akamatsu, K.; Takeda, Y. Polyglutamation of a novel antifolate, MX-68, is not necessary for its anti-arthritic effect. Eur. J. Pharmacol. 2002, 435, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Toshiaki, T.; Matsumoto, J.; Tohma, T.; Kanke, T.; Wada, Y.; Nagao, M.; Inagaki, N.; Nagai, H.; Zhang, M.; Timmerman, H. VUF-K-8788, a Periphery-Selective Histamine H1 Antagonist with Anti-pruritic Activities. Jpn. J. Pharmacol. 2001, 86, 55–64. [Google Scholar] [CrossRef]
- Ajani, O.O. Functionalized 1,4-Benzothiazine: A Versatile Scaffold with Diverse Biological Properties. Arch. Pharm. 2012, 345, 841–851. [Google Scholar] [CrossRef]
- Fringuelli, R.; Milanese, L.; Schiaffella, F. Role of 1,4-Benzothiazine Derivatives in Medicinal Chemistry. Mini-Rev. Med. Chem. 2005, 5, 1061–1073. [Google Scholar] [CrossRef]
- Rai, A.; Singh, A.K.; Raj, V.; Saha, S. 1,4-Benzothiazines-A Biologically Attractive Scaffold. Mini-Rev. Med. Chem. 2018, 18, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Bakavoli, M.; Nikpour, M.; Rahimizadeh, M.; Saberi, M.R.; Sadeghian, H. Design and synthesis of pyrimido[4,5-b][1,4]benzothiazine derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2007, 15, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Nami, N.; Oskooie, H.A.; Hekmatshoar, R. Microwave-assisted synthesis of quinoxalines, benzoxazines, and benzothiazines under solvent-fee conditions. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1873–1878. [Google Scholar] [CrossRef]
- Melkonyan, F.; Topolyan, A.; Karchava, A.; Yurovskaya, M. A general synthesis of N-substituted 1, 4-benzoxazine-and 1,4-benzothiazine-2-carboxylates via copper-catalyzed intramolecular amination of arylbromides. Tetrahedron 2011, 67, 6826–6832. [Google Scholar] [CrossRef]
- Pi, H.J.; Liu, H.; Du, W.; Deng, W.P. The facile synthesis of benzothiazolylideneacetates and 1,4-benzothiazines through a highly controllable oxidation of benzothiazolylacetates. Tetrahedron Lett. 2009, 50, 4529–4531. [Google Scholar] [CrossRef]
- Adib, M.; Sheibani, E.; Zhu, L.G.; Bijanzadeh, H.R. A novel reaction between benzothiazoles and diaroylacetylenes in the presence of Meldrum’s acid: Ring expansion of benzothiazoles to functionalized 1,4-benzothiazines. Tetrahedron Lett. 2009, 50, 4420–4422. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S.; Rostovskii, N.V. Advances in 2H-azirine chemistry: A seven-year update. Tetrahedron 2019, 75, 2555–2624. [Google Scholar] [CrossRef]
- Xu, F.; Zeng, F.-W.; Luo, W.-J.; Zhang, S.-Y.; Huo, J.-Q.; Li, Y.-P. 2H-Azirines: Recent Progress in Synthesis and Applications. Eur. J. Org. Chem. 2024, 27, e202301292. [Google Scholar] [CrossRef]
- Charushin, V.N.; Verbitskiy, E.V.; Chupakhin, O.N.; Vorobyeva, D.V.; Gribanov, P.S.; Osipov, S.N.; Ivanov, A.V.; Martynovskaya, S.V.; Sagitova, E.F.; Dyachenko, V.D.; et al. The chemistry of heterocycles in the 21st century. Russ. Chem. Rev. 2024, 93, RCR5125. [Google Scholar] [CrossRef]
- Rostovskii, N.V.; Agafonova, A.V.; Smetanin, I.A.; Novikov, M.S.; Khlebnikov, A.F.; Ruvinskaya, J.O.; Starova, G.L. Metal-Catalyzed Isomerization of 5-Heteroatom-Substituted Isoxazoles as a New Route to 2-Halo-2H-azirines. Synthesis 2017, 49, 4478–4488. [Google Scholar] [CrossRef]
- Filippov, I.P.; Agafonova, A.V.; Titov, G.D.; Smetanin, I.A.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of Imidazo[1,2-a]pyridines via Near UV Light-Induced Cyclization of Azirinylpyridinium Salts. J. Org. Chem. 2022, 87, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippov, I.P.; Agafonova, A.V.; Rostovskii, N.V.; Novikov, M.S. Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate. Molbank 2025, 2025, M2101. https://doi.org/10.3390/M2101
Filippov IP, Agafonova AV, Rostovskii NV, Novikov MS. Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate. Molbank. 2025; 2025(4):M2101. https://doi.org/10.3390/M2101
Chicago/Turabian StyleFilippov, Ilya P., Anastasiya V. Agafonova, Nikolai V. Rostovskii, and Mikhail S. Novikov. 2025. "Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate" Molbank 2025, no. 4: M2101. https://doi.org/10.3390/M2101
APA StyleFilippov, I. P., Agafonova, A. V., Rostovskii, N. V., & Novikov, M. S. (2025). Methyl (1aRS,7aSR)-7-formyl-1a-phenyl-1,1a-dihydroazirino[2,3-b]benzo[e][1,4]thiazine-7a(7H)-carboxylate. Molbank, 2025(4), M2101. https://doi.org/10.3390/M2101






