Abstract
Triphenylamine and phenol derivatives are two types of antimicrobial molecules with broad application prospects. Through functional modification, these compounds integrate fluorescence imaging and photodynamic antibacterial therapy, thereby achieving theranostic capabilities. They exert broad-spectrum and highly efficient antimicrobial activity via a membrane-disrupting mechanism, which consequently reduces the propensity for inducing drug resistance. In this work, triphenylamine-phenol derivatives (TPO) were designed and synthesized through three consecutive reactions: Wittig reaction, Heck reaction, and substitution reaction. Double bonds, hydroxyl groups, and brominated alkyl chains were gradually introduced to finally obtain the target product (E)-4-(4-((8-bromooctyl)oxy)styryl)-N,N-diphenylaniline (5). This study provides new insights into the development of novel and highly efficient antibacterial agents.
1. Introduction
The increasing frequency of bacterial resistance, coupled with the global antibiotic crisis, highlights the urgent need for innovation in antimicrobial research [1]. In this context, there is an imperative to develop antimicrobial molecules with novel structures, unique mechanisms of action, and a low propensity to induce drug resistance [2]. Therefore, exploring new antimicrobial core scaffolds is considered a crucial strategy for overcoming existing limitations and developing next-generation antimicrobial therapies.
Phenol is characterized by a hydroxyl group directly attached to a benzene ring (Figure 1I). It gained prominence after serendipitously discovering its efficacy in disinfecting surgical instruments, leading to its widespread use as a potent surgical antiseptic. Moreover, the phenoxy group is extensively incorporated into various bioactive molecules, such as the disinfectant domiphen and the antibiotic penicillin V (Figure 1II,III). Based on this structural feature, our research group previously introduced the phenoxy group as an aromatic core into the design of quaternary ammonium salt-based small-molecule antimicrobial peptide mimics, successfully obtaining compounds with promising antibacterial activity (Figure 1IV,V) [3,4]. Therefore, phenol derivatives are considered ideal structural motifs for constructing multifunctional antibacterial agents.
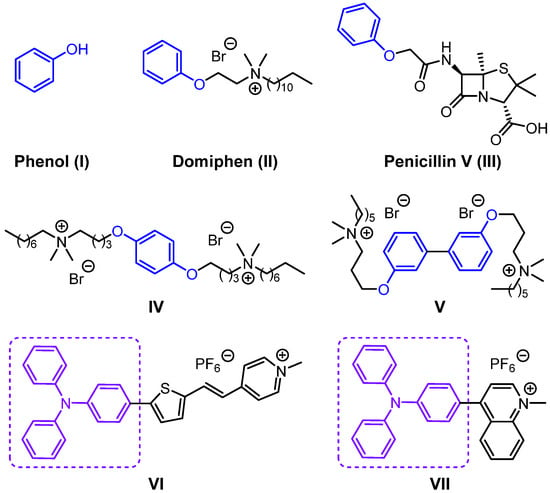
Figure 1.
Chemical structures of the phenoxy-containing antibacterial agents and triphenylamine derivatives.
Triphenylamine (TPA) is a tertiary amine with a central nitrogen atom bonded to three benzene rings [5]. The central nitrogen atom is coplanar with the three adjacent carbon atoms, and the three benzene rings adopt a propeller-like arrangement. The TPA moiety exhibits high hydrophobicity and a significant hyperconjugative electronic effect; its propeller-like twisted configuration and strong electron-donating ability make it highly favorable for constructing photoactive fluorescent materials [6,7,8]. In recent years, a series of D−π−A (donor−π−acceptor) structures based on triphenylamine derivatives have been reported. Amongst these, pyridine-substituted triphenylamine derivatives have been the most extensively studied and are widely used in the fields of bacterial detection, imaging, and elimination [9,10,11,12,13]. Tang et al. used pyridinium cation as the electron acceptor and triphenylamine as the electron donor to synthesize triphenylamine derivatives (with a D-π-A structure) (Figure 1VI,VII). Therefore, triphenylamine is regarded as an ideal building block for constructing multifunctional antimicrobial agents.
In this work, aiming at the development of highly efficient antibacterial agents, triphenylamine-phenol derivatives (TPO) were designed and synthesized, which provides a new strategy for the design of antibacterial agents.
2. Results and Discussion
Following the previously reported synthetic route for pyridinium cations [9], piperidine was used as the base, but the expected product was not obtained (Scheme 1). Therefore, it is speculated that this result is due to the insufficient electron-withdrawing ability of p-cresol, which leads to the low reactivity of the benzene ring.
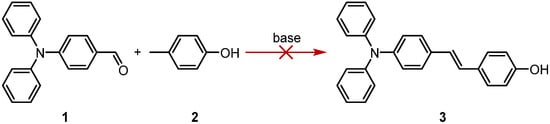
Scheme 1.
Attempts on the synthetic route of (E)-4-(4-(diphenylamino)styryl)phenol.
To guide the design of the next experiments, a comprehensive review of the literature was conducted [14]. With 4-(diphenylamino) benzaldehyde (compound 1) as the starting material, a Wittig reaction was carried out in N,N-dimethylformamide (DMF) using methyltriphenylphosphine iodide and potassium tert-butoxide as the Wittig reagent and base, respectively. The reaction gave 4-(diphenylamino)styrene (compound 4), which provided the key carbon–carbon double bond for the subsequent Heck coupling reaction. A Heck reaction was then carried out with p-bromophenol in DMF at 90 °C for 10 h, using palladium acetate as the catalyst, tris(o-tolyl)phosphine as the ligand, and triethylamine as the base. In this reaction, the carbon-carbon double bond of 4-(diphenylamino)styrene coupled with the brominated benzene ring of p-bromophenol, and a hydroxyl group (-OH) was introduced simultaneously to generate an intermediate 3 containing both a double bond and a hydroxyl group. This intermediate provides a hydroxyl site for subsequent substitution reactions (Scheme 2).
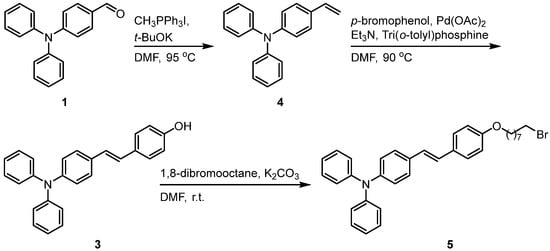
Scheme 2.
Synthetic Route of (E)-4-(4-((8-bromooctyl)oxy)styryl)-N,N-diphenylaniline (5).
Subsequently, the hydroxyl group in the intermediate 3 (Scheme 2) molecule acted as a nucleophile, attacking the brominated carbon atom of the 1,8-dibromooctane molecule and displacing the bromide atom. This nucleophilic substitution reaction formed an ether bond and simultaneously introduced a brominated alkyl chain, thereby affording the final compound 5.
This synthetic route employed a stepwise construction strategy, in which functional groups and structural motifs were installed sequentially. Starting from a simple aldehyde, a carbon–carbon double bond, a hydroxyl functional group, and a brominated alkyl chain were sequentially introduced; each transformation provided the desired structural control for subsequent reactions.
3. Materials and Methods
3.1. Chemicals and Instrumentations
Unless otherwise specified, all reagents used were of analytical grade. The key reagents included: 4-(diphenylamino)benzaldehyde, p-cresol, methyltriphenylphosphonium iodide, potassium tert-butoxide, N,N-dimethylformamide, p-bromophenol, palladium acetate, tris(o-tolyl)phosphine, triethylamine, 1,8-dibromooctane, and potassium carbonate. The main instruments were as follows: Thermostatic heating magnetic stirrer (Model DF-101S), manufactured by Shanghai Yukang Science and Education Instrument Equipment Co., Ltd. (Shanghai, China), Rotary evaporator (Model N-1100V), obtained from EYELA (Tokyo Rika Kikai Co., Ltd., Tokyo, Japan); Chemical diaphragm pump (Model MZ 2C NT), product of Vacuubrand GmbH, Wertheim, Germany; Vacuum drying oven (Model DZF-6020), supplied by Gongyi Jinghua Instrument Co., Ltd. (Henan, China); Circulating water vacuum pump (Model SHZ-D(III)), produced by Zhengzhou Yuxiang Instrument Equipment Co., Ltd. (Henan, China); Electronic balance (Model ME203E), brand of Mettler Toledo, Zurich, Switzerland; Triple-purpose UV analyzer (Model ZF-7), manufactured by Shanghai Kanghua Biochemical Instrument Co., Ltd. (Shanghai, China); Ultrasonic cleaner (Model KQ5200), obtained from Kunshan Ultrasonic Instrument Co., Ltd. (Jiangsu, China); Automatic image melting point apparatus (Model SGW®-650), produced by Shanghai Yidian Physical Optics Instrument Co., Ltd. (Shanghai, China); High-resolution quadrupole-time-of-flight mass spectrometer (Model Q-TofMicro), product of Waters-Micromass Co., Ltd., Milford, MA, USA; Superconducting nuclear magnetic resonance spectrometer (Model DPX-400), manufactured by Bruker Corporation, Ettlingen, Germany.
3.2. N,N-Diphenyl-4-vinylaniline (4)
To a solution of 4-(diphenylamino)benzaldehyde (3.00 g, 10.98 mmol, 1.00 equiv) and methyltriphenylphosphonium iodide (5.04 g, 12.48 mmol, 1.14 equiv) in DMF (30 mL), a DMF solution (45 mL) of potassium tert-butoxide (1.46 g, 12.99 mmol, 1.18 equiv) was added dropwise, stirred at room temperature for 30 min, and then heated to 95 °C for 13 h. After completion of the reaction, the mixture was extracted with water and dichloromethane (DCM). The organic layer was washed successively with water (80 mL) and 1 M HCl. The combined organic layer was dried over anhydrous Na2SO4, filtered, concentrated and then purified the crude product by column chromatography (eluent: dichloromethane: Petroleum ether = 1:7, v/v) to obtain intermediate 4 (2.73 g) as a white solid, with a yield of 91%. 1H NMR (400 MHz, Chloroform-d) δ 7.22–7.10 (m, 6H), 7.04–6.96 (m, 4H), 6.96–6.87 (m, 4H), 6.56 (dd, J = 17.6, 10.9 Hz, 1H), 5.54 (dd, J = 17.6, 1.0 Hz, 1H), 5.05 (dd, J = 10.8, 1.0 Hz, 1H); 13C NMR (101 MHz, Chloroform-d) δ 147.74, 147.61, 136.34, 132.00, 129.38, 127.19, 124.50, 123.75, 123.05, 112.28.
3.3. (E)-4-(4-(Diphenylamino)styryl)phenol (3)
In a three-necked round-bottomed flask (50 mL), intermediate 4 (2 g, 7.37 mmol, 1.00 eq.), 4-bromophenol (1.28 g, 7.37 mmol, 1.00 eq.), palladium acetate (0.17 g, 0.74 mmol, 0.1 eq.) and o-trimethylphenylphosphine (0.45 g, 1.47 mmol, 0.2 eq.) were dissolved in a mixed solution consisting of TEA/DMF (4:1, v/v, 15 mL) and triethylamine (2.05 mL, 14.74 mmol, 2.00 eq.). Under nitrogen protection, the reaction mixture was heated to 90 °C and stirred for 6 h. After completion of the reaction, the mixture cooled to room temperature, and then extracted with water (30 mL) and DCM (30 mL). The combined organic phases were washed with water (30 mL) three times, then washed with saturated sodium chloride solution (10 mL) 3 times, dried over anhydrous Na2SO4, filtered, and concentrated by vacuum rotary evaporation. The crude product was purified by silica gel column chromatography (eluent: dichloromethane: petroleum ether = 2:1, v/v) to afford intermediate 3 (0.99 g) as a yellow solid with a yield of 50%. 1H NMR (400 MHz, Chloroform-d) δ 7.36 (dd, J = 10.2, 7.7 Hz, 4H), 7.29–7.19 (m, 4H), 7.16–6.97 (m, 8H), 6.92 (d, J = 2.9 Hz, 2H), 6.85–6.76 (m, 2H). 13C NMR (101 MHz, Chloroform-d) δ 163.32, 155.91, 147.75, 147.02, 132.20, 130.18, 129.40, 127.81, 127.19, 126.99, 125.91, 124.46, 123.99, 123.01, 115.87, 37.02, 31.96.
3.4. (E)-4-(4-((8-Bromooctyl)oxy)styryl)-N,N-diphenylaniline (5)
In a round-bottomed flask (50 mL), intermediate 3 (2 g, 5.5 mmol, 1.00 eq.), 1,8-dibromooctane (0.72 mL, 7.15 mmol, 1.30 eq.), and potassium carbonate (1.52 g, 11.01 mmol, 2 eq.) were dissolved in N,N-dimethylformamide (DMF, 15 mL). The reaction mixture was stirred at room temperature for 24 h. After completion of the reaction, the mixture cooled to room temperature, and then extracted with water (20 mL) and DCM (20 mL). The combined organic phase was washed with water (3 × 20 mL) and saturated NaCl solution (3 × 10 mL), then dried over anhydrous Na2SO4. After filtration, the mixture was concentrated by rotary evaporation under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: dichloromethane: petroleum ether = 1:5, v/v), yielding product 5 (0.88 g) as a pale yellow solid with a yield of 44%. 1H NMR (400 MHz, Chloroform-d) δ 7.38 (dd, J = 21.5, 8.7 Hz, 4H), 7.26 (s, 2H), 7.22 (s, 2H), 7.13–7.07 (m, 4H), 7.05–6.98 (m, 4H), 6.96–6.84 (m, 4H), 3.96 (s, 2H), 3.41 (d, J = 6.7 Hz, 2H), 1.89–1.65 (m, 4H), 1.49–1.32 (m, 8H). 13C NMR (101 MHz, Chloroform-d) δ 158.74, 147.72, 147.03, 132.13, 130.35, 129.36, 127.59, 127.16, 126.85, 126.08, 124.45, 123.95, 122.99, 114.79, 68.07, 34.12, 32.88, 29.33, 29.30, 28.80, 28.20, 26.06. HRMS (ESI) Calculated for C34H36BrNO [M − H]−: 552.1902, found: 552.1898.
4. Conclusions
In summary, this work focused on the synthesis of a triphenylamine-phenol derivative. The target molecule, (E)-4-(4-((8-bromooctyl)oxy)styryl)-N,N-diphenylaniline, was successfully obtained via a three-step reaction sequence comprising the Wittig, Heck, and nucleophilic substitution reactions. Its structure was unambiguously confirmed by 1H NMR, 13C NMR, and high-resolution mass spectrometry (HRMS) spectroscopy. We anticipate that this scaffold can serve as a core structural motif for developing novel and highly efficient antibacterial agents.
Supplementary Materials
The following supporting information can be downloaded online: 1H NMR spectrum, 13C NMR spectrum and HRMS spectrum of compound 5.
Author Contributions
Conceptualization, Y.-Q.S.; Software, C.D.K.A.; investigation, Y.-N.W.; Formal Analysis, M.A.-W.; Data Curation, K.-W.Z. and R.L.; writing—original draft preparation, Y.-Q.S.; writing—review and editing, E.Z. and Y.-H.Z.; supervision, E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Science and Technology of Henan Province (No. 252102311218) and the Open Grant from the Pingyuan Laboratory (2023PY-OP-0103).
Data Availability Statement
The data are contained within this article and the Supplementary Materials.
Acknowledgments
We gratefully acknowledge Saiya Yang and Yuyang Ma for valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zheng, M.; Cai, J. Small molecules with membrane-active antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 21292–21299. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Yang, Y.; Cai, J.; Kong, H.; Bai, M.; Fu, X.; Qin, S.; Zhang, E. Synthesis and bioactivities of new membrane-active agents with aromatic linker: High selectivity and broad-spectrum antibacterial activity. ACS Infect. Dis. 2019, 5, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Chen, S.; Ampomah-Wireko, M.; Gao, C.; Xia, Z.; Nininahazwe, L.; Qin, S.; Zhang, E. Development of biaromatic core-linked antimicrobial peptide mimics: Substituent position significantly affects antibacterial activity and hemolytic toxicity. Eur. J. Med. Chem. 2022, 247, 115029. [Google Scholar] [CrossRef] [PubMed]
- Karak, A.; Manna, S.K.; Mahapatra, A.K. Triphenylamine-based small-molecule fluorescent probes. Anal. Methods 2022, 14, 972–1005. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhao, Z.; Cheng, D. Recent progress on triphenylamine materials: Synthesis, properties, and applications. Mol. Cryst. Liq. Cryst. 2017, 648, 223–235. [Google Scholar] [CrossRef]
- Xu, Z.; Luo, Y.; Yang, X.; Ren, Y.; Liu, G.; Zhang, M.-X. Synthesis and properties of D-π-A triphenylamine derivatives with solvatochromism and bioimaging application. J. Photochem. Photobiol. A Chem. 2023, 444, 115002. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, D.; Yang, J.; Fang, M.; Li, Z. Tunable photoresponsive behaviors based on triphenylamine derivatives: The pivotal role of π-conjugated structure and corresponding application. Adv. Mater. 2021, 33, 2104002. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Zhou, C.; Wu, S.; Yu, B.; Zhang, Z.; Song, N.; Lee, M.M.S.; Xu, W.; Xu, F.-J.; Wang, D.; et al. Evaluation of structure-function relationships of aggregation-induced emission luminogens for simultaneous dual applications of specific discrimination and efficient photodynamic killing of gram-positive bacteria. J. Am. Chem. Soc. 2019, 141, 16781–16789. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.S.; Yu, E.Y.; Yan, D.; Chau, J.H.C.; Wu, Q.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Wang, D.; Tang, B.Z. The role of structural hydrophobicity on cationic amphiphilic aggregation-induced emission photosensitizer-bacterial interaction and photodynamic efficiency. ACS Nano 2023, 17, 17004–17020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Xia, F.-W.; Wang, Y.; Shi, H.-Z.; Wang, L.-J.; Zhao, Y.; Song, J.-X.; Wu, M.-Y.; Feng, S. Molecular charge and antibacterial performance relationships of aggregation-induced emission photosensitizers. ACS Appl. Mater. Interfaces 2023, 15, 17433–17443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhuang, Z.; Xing, L.; Li, J.; Yang, Z.; Ji, S.; Hu, R.; Zhao, Z.; Huo, Y.; Tang, B.Z. The AIE-active dual-cationic molecular engineering: Synergistic effect of dark toxicity and phototoxicity for anticancer therapy. Adv. Funct. Mater. 2021, 31, 2106988. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Zhuang, Z.; Wang, B.; Dai, J.; Feng, G.; Lou, X.; Xia, F.; Zhao, Z.; Tang, B.Z. Effective therapy of drug-resistant bacterial infection by killing planktonic bacteria and destructing biofilms with cationic photosensitizer based on phosphindole oxide. Small 2022, 18, 22007439. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, M.; Li, Z.-H.; Qu, Y.; Li, Y.; Ampomah-Wireko, M.; Li, D.; Kong, H.; Wu, Y.; Hossain, A.A.; et al. Important role of triphenylamine in modulating the antibacterial performance relationships of antimicrobial peptide mimics by alkyl chain engineering. J. Med. Chem. 2025, 68, 10299–10313. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).