(1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol
Abstract
1. Introduction
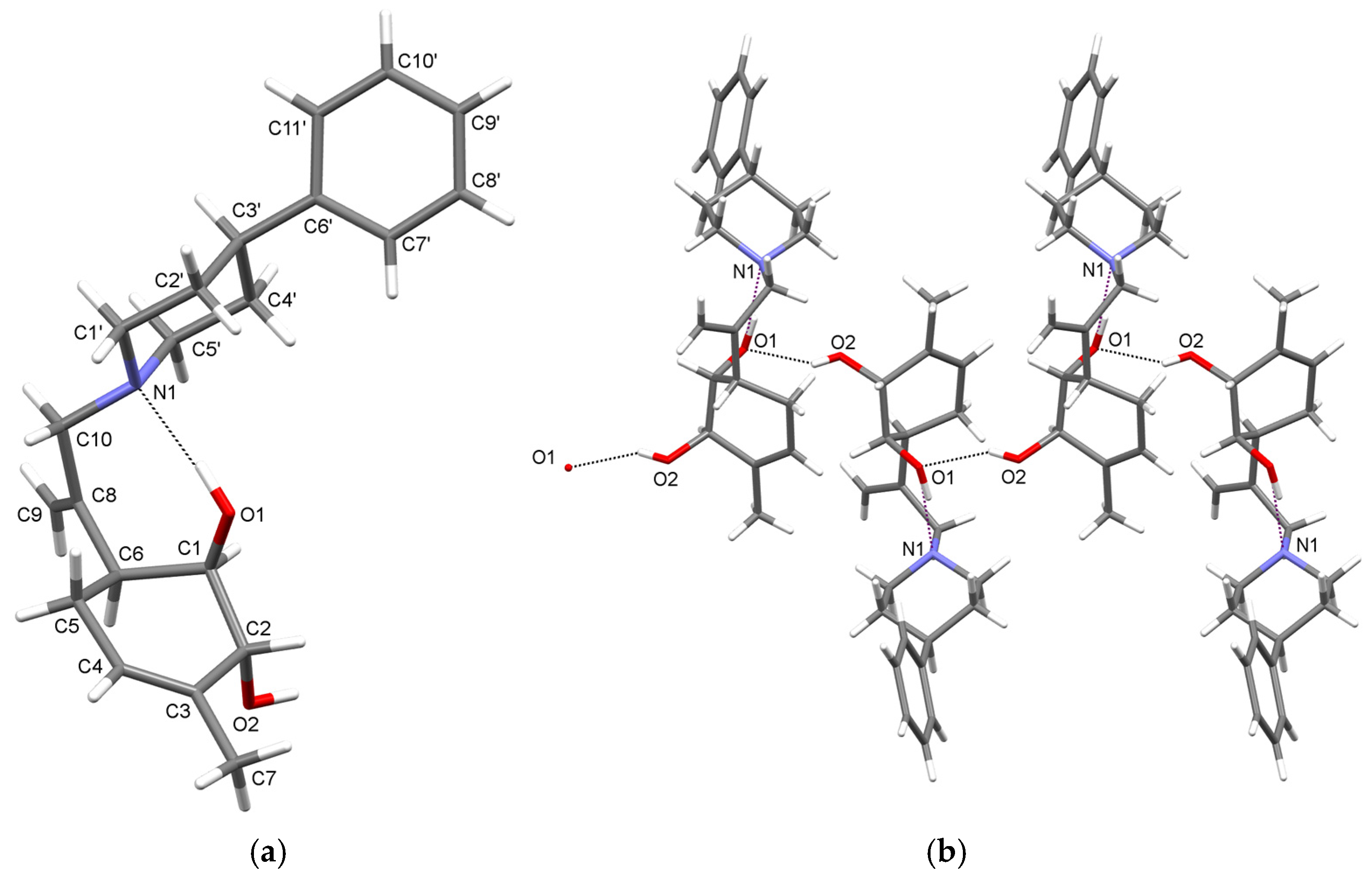
2. Results and Discussion
3. Materials and Methods
Synthesis of (1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol (2)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Sheng, M.; Hanson, J.E. Targeting synapse function and loss for treatment of neurodegenerative diseases. Nat. Rev. Drug Discov. 2024, 23, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Westenberger, A. Genetics of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a008888. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Del Tredici, K.; Braak, H. Review: Sporadic Parkinson’s disease: Development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016, 42, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Koeglsperger, T.; Rumpf, S.L.; Schließer, P.; Struebing, F.L.; Brendel, M.; Levin, J.; Trenkwalder, C.; Höglinger, G.U.; Herms, J. Neuropathology of incidental Lewy body & prodromal Parkinson’s disease. Mol. Neurodegener. 2023, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Chopade, P.; Chopade, N.; Zhao, Z.; Mitragotri, S.; Liao, R.; Chandran Suja, V. Alzheimer’s and Parkinson’s disease therapies in the clinic. Bioeng. Transl. Med. 2023, 8, e10367. [Google Scholar] [CrossRef] [PubMed]
- di Biase, L.; Pecoraro, P.M.; Carbone, S.P.; Caminiti, M.L.; Di Lazzaro, V. Levodopa-Induced Dyskinesias in Parkinson’s Disease: An Overview on Pathophysiology, Clinical Manifestations, Therapy Management Strategies and Future Directions. J. Clin. Med. 2023, 12, 4427. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Il’ina, I.V.; Morozova, E.A.; Korchagina, D.V.; Karpova, E.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Highly Potent Activity of (1R,2R,6S)-3-Methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in Animal Models of Parkinson’s Disease. J. Med. Chem. 2011, 54, 3866–3874. [Google Scholar] [CrossRef] [PubMed]
- Valdman, E.; Kapitsa, I.; Ivanova, E.; Voronina, T.; Ardashov, O.; Volcho, K.; Khazanov, V.; Salakhutdinov, N. Evolution of anti-parkinsonian activity of monoterpenoid (1R,2R,6S)-3- methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol in various in vivo models. Eur. J. Pharmacol. 2017, 815, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Korchagina, D.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Antiparkinsonian activity of some 9-N-, O-, S- and C-derivatives of 3-methyl-6-(prop-1-en-2-yl)cyclohex-3-ene-1,2-diol. Bioorg. Med. Chem. 2013, 21, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Podturkina, A.V.; Ardashov, O.V.; Soldatova, Y.V.; Poletaeva, D.A.; Smolina, A.V.; Vasyuchenko, E.P.; Vyatkin, Y.V.; Li-Zhulanov, N.S.; Faingold, I.I.; Salakhutdinov, N.F.; et al. Inhibitory Activity of N- and S-Functionalized Monoterpene Diols Towards Monoamine Oxidases A and B. Int. J. Mol. Sci. 2025, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- SADABS, v. 2016-2; Bruker AXS: Madison, WI, USA, 2016.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D.J. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, C71, 3–8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podturkina, A.V.; Li-Zhulanov, N.S.; Rybalova, T.V.; Volcho, K.P.; Salakhutdinov, N.F. (1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol. Molbank 2025, 2025, M2088. https://doi.org/10.3390/M2088
Podturkina AV, Li-Zhulanov NS, Rybalova TV, Volcho KP, Salakhutdinov NF. (1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol. Molbank. 2025; 2025(4):M2088. https://doi.org/10.3390/M2088
Chicago/Turabian StylePodturkina, Alexandra V., Nikolai S. Li-Zhulanov, Tatyana V. Rybalova, Konstantin P. Volcho, and Nariman F. Salakhutdinov. 2025. "(1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol" Molbank 2025, no. 4: M2088. https://doi.org/10.3390/M2088
APA StylePodturkina, A. V., Li-Zhulanov, N. S., Rybalova, T. V., Volcho, K. P., & Salakhutdinov, N. F. (2025). (1R,2R,6S)-3-Methyl-6-(3-(4-phenylpiperidin-1-yl)prop-1-en-2-yl)cyclohex-3-ene-1,2-diol. Molbank, 2025(4), M2088. https://doi.org/10.3390/M2088







