2-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-2-ium Iodide
Abstract
1. Introduction
2. Results
2.1. Synthesis
2.2. Spectroscopic Characterization
- IR (KBr): 2918, 2844, 1675, 1452, 1435, 1316 cm−1.
- 1H-NMR (CDCl3, 400 MHz): δ = 4.34 (s, 3H, CH3, satellites: 1JC-H 147 Hz), 3.57 (‘t’, 2 H, 3JH-H = 6 Hz, H2C-9), 3.04 (t, 2 H, 3JH-H = 6 Hz, H2C-4), 1.86 (qui, 2 H, 3JH-H = 6 Hz, H2C-8), 1.69 (qui, 2 H, 3JH-H = 6 Hz, H2C-4), 1.44 (qui, 2 H, 3JH-H = 6 Hz, H2C-6), 1.29 (qui, 2 H, 3JH-H = 6 Hz, H2C-7).
- 13C-NMR (CDCl3, 100 MHz): δ = 173.2 (C-9a; satellites: 1JC-Se: 158.4 Hz), 158.4 (C-3a; satellites: 2JC-Se: 51 Hz), 47.3 (CH3, satellites: 2JC-Se: 84.3 Hz), 33.8 (C-9), 30.9 (C-8), 30.5 (C.5), 28.0 (C-4), 26.1 (C-6), 24.7 (C-7).
- 77Se-NMR (CDCl3, 76.3 MHz, Me2Se): δ = 1307.5.
- 15N-NMR: (CDCl3, 60.8 MHz): δ = 275.68, 410.26.
- Mass Spectrometry: (field desorption): 587 (5%, Se2-pattern, M2-I−), 231.2 (100%, Se-pattern, M-I−), 142 (CH3I).
- UV-Vis: Solutions of the title compound in ethanol displayed three maxima in the UV-Vis region: λ = 423 nm (ε = 1388 L/mol × cm−1), λ = 290 nm (ε = 25,311 L/mol × cm−1), and λ = 254 nm (ε = 26,609 L/mol × cm−1), significantly different from the 3-methylselenadiazolium isomer: λ = 431 nm (ε = 1820 L/mol × cm−1) and l = 269 nm (e = 19,353 L/mol × cm−1). Upon continued exposure to UV, the compounds decompose.
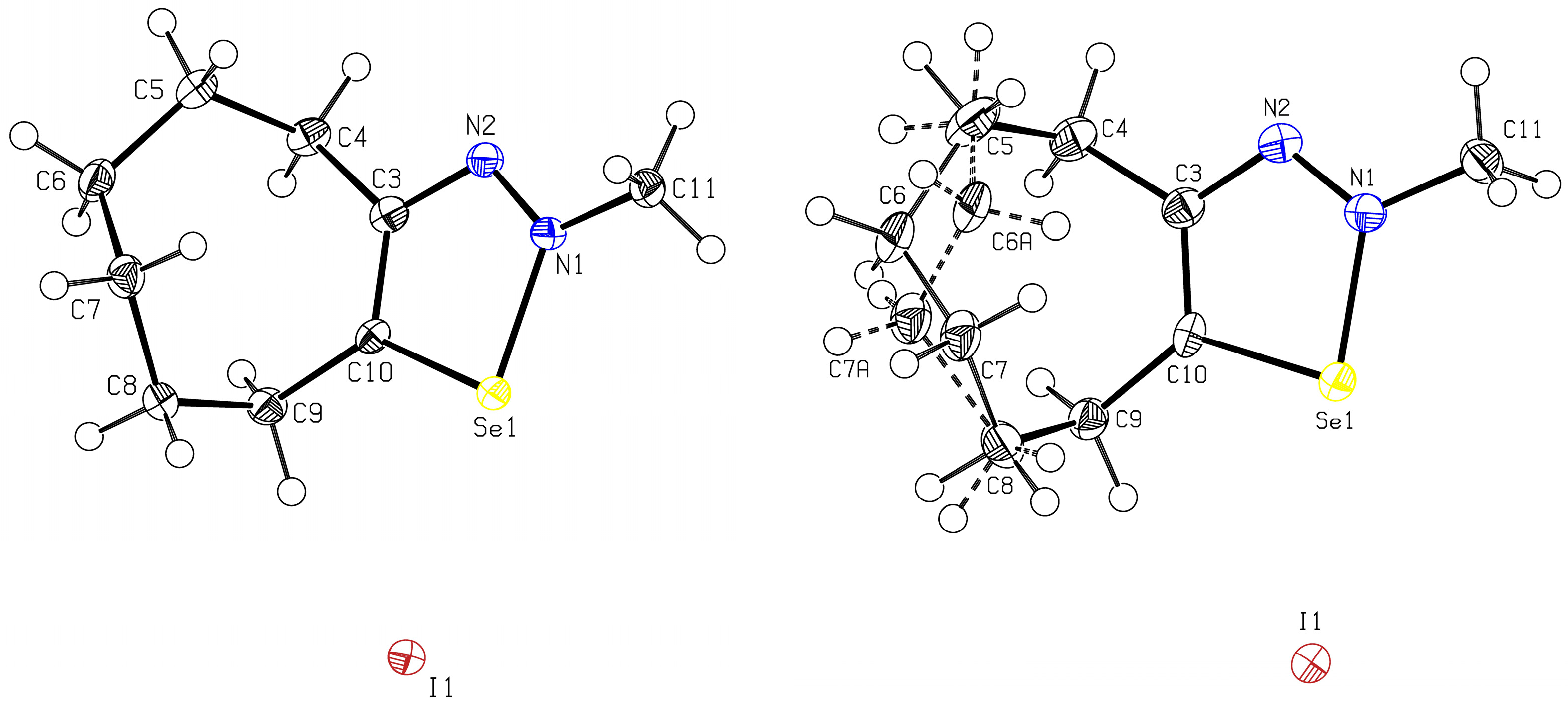
2.3. Crystal Structure
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bissinger, H.-J.; Detert, H.; Meier, H. 1-Cycloundecen-3-in. Liebigs Ann. Chem. 1988, 1988, 221–224. [Google Scholar] [CrossRef]
- Detert, H.; Meier, H. trans- and cis-Bicyclo[n.1.0]alk-2-ynes of Medium Ring Size. Liebigs Ann. 1997, 1997, 1557–1564. [Google Scholar] [CrossRef]
- Gleiter, R.; Kratz, D.; Schäfer, W.; Schehlmann, V.J. Synthesis and properties of medium-sized (C9–C12) carbocyclic diacetylenes. Am. Chem. Soc. 1991, 113, 9258–9264. [Google Scholar] [CrossRef]
- Gleiter, R.; Karcher, M.; Jahn, R.; Irngartinger, H. Darstellung, Struktur und Eigenschaften von 1,6-Cyclodecadiin. Vergleich mit 1,5-Cyclooctadiin und 1,7-Cyclododecadiin. Chem. Berichte 1988, 121, 735–740. [Google Scholar] [CrossRef]
- Meier, H.; Mayer, W.; Kolshorn, H. Synthese von 9-Oxabicyclo[6.1.0]non-3-in. Chem. Berichte 1987, 120, 685–689. [Google Scholar] [CrossRef]
- Gleiter, R.; Langer, H.; Schehlmann, V.; Nuber, B. Stepwise Approach to Metal-Capped 4-Fold-Bridged Cyclobutadienophanes. Organometallics 1995, 14, 975–986. [Google Scholar] [CrossRef]
- Mhaidat, N.M.; Al-Smadi, M.; Al-Momani, F.; Alzoubi, K.H.; Mansi, I.; Al-Balas, Q. Synthesis, antimicrobial and in vitro antitumor activities of a series of 1,2,3-thiadiazole and 1,2,3-selenadiazole derivatives. Drug Des. Dev. Ther. 2015, 9, 3645–3652. [Google Scholar] [CrossRef]
- Al-Smadi, M.L.; Mansour, R.; Mahasneh, A.; Khabour, O.F.; Masadeh, M.M.; Alzoubi, K.H. Synthesis, Characterization, Antimicrobial Activity, and Genotoxicity Assessment of Two Heterocyclic Compounds Containing 1,2,3-Selena- or 1,2,3-Thiadiazole Rings. Molecules 2019, 24, 4082. [Google Scholar] [CrossRef]
- Jadhav, A.A.; Dhanwe, V.P.; Joshi, P.G.; Khanna, P.K. Solventless synthesis of new 4,5-disubstituted 1,2,3-selenadiazole derivatives and their antimicrobial studies. Cogent Chem. 2016, 2, 1144670. [Google Scholar] [CrossRef]
- Joshi, P.G.; More, M.S.; Jadhav, A.A.; Khanna, P.K. Materials and biological applications of 1,2,3-selenadiazoles: A review. Mater. Today Chem. 2000, 16, 100255. [Google Scholar] [CrossRef]
- Nunn, A.J.; Ralph, J.T.J. Quaternisation of 2,1,3-benzothiadiazole and 2,1,3-benzoselenadiazole. Part I. Preparation of methyl- and ethyl-2,1,3-benzothiadiazolium and -benzoselenadiazolium salts. Chem. Soc. 1965, 1965, 6769–6777. [Google Scholar] [CrossRef]
- Ramadoss, V.; Alonso-Castro, A.J.; Campos-Xolalpa, N.; Ortiz-Alvarado, R.; Yahuaca-Juarez, B.; Solorio-Alvarado, C.R. Total synthesis of kealiiquinone: The regio-controlled strategy for accessing its 1-methyl-4-arylbenzimidazolone core. RSC Adv. 2018, 8, 30761–30776. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Hokelek, T.; Mammadova, G.Z.; Hasanov, K.I.; Javadzade, T.A.; Belay, A.N. Synthesis, crystal structure, Hirshfeld surface and crystal void analysis of 4-fluoro-benzo[c][1,2,5]selena-diazol-1-ium chloride. Acta Cryst. E 2025, 81, 252–256. [Google Scholar] [CrossRef]
- Lee, L.M.; Corless, V.; Luu, H.; He, A.; Jenkins, H.; Britten, J.F.; Adam, P.F.; Vargas-Baca, I. Synthetic and structural investigations of bis(N-alkyl-benzoselenadiazolium) cations. Dalton Trans. 2019, 48, 12541–12548. [Google Scholar] [CrossRef]
- Artemjev, A.A.; Sapronov, A.A.; Kubasov, A.S.; Peregudov, A.S.; Novikov, A.S.; Egorov, A.R.; Khrustalev, V.N.; Borisov, A.V.; Matsulevich, Z.V.; Shikhaliyev, N.G.; et al. Diverse Cyclization Pathways Between Nitriles with Active α-Methylene Group and Ambiphilic 2-Pyridylselenyl Reagents Enabled by Reversible Covalent Bonding. Int. J. Mol. Sci. 2024, 25, 12798. [Google Scholar] [CrossRef]
- Jaffari, G.A.; Nunn, A.J.; Ralph, J.T.J. 1,2,3-Benzothiadiazole. Part VI. Investigations on the quaternisation of 1,2,3-benzothiadiazole and 1,2,3-benzoselenadiazole. Chem. Soc. C 1970, 15, 2060–2062. [Google Scholar] [CrossRef]
- Gil, M.J.; Reliquet, A.; Reliquet, F.; Meslin, J.C. Synthese et proprietes de 2-hydrazonophenyl-selenoacetamides. Phosphorus Sulfur Silicon Relat. Elem. 2000, 164, 161–172. [Google Scholar] [CrossRef]
- Butler, R.N.; Fox, A.J. Transformations of selenadiazoliumyl-N-unsubstituted methanides (ylides) to new divinyl selenide derivatives and substituted 1,3,5-selenadiazines. Organoselenium systems from azolium 1,3-dipoles. Chem. Soc. Perkin Trans. 2001, 1, 394–397. [Google Scholar] [CrossRef]
- Srivastava, K.; Chakraborty, T.; Singh, H.B.; Butcher, R.J. Intramolecularly coordinated azobenzene selenium derivatives: Effect of strength of the Se⋯N intramolecular interaction on luminescence. Dalton Trans. 2011, 40, 4489–4496. [Google Scholar] [CrossRef]
- Majeed, Z.; McWhinnie, W.R.; Lowe, P.R. A T-shaped selenenyl halide. Acta Cryst. C 2000, C56, e105–e106. [Google Scholar] [CrossRef]
- Reid, D.H.; Rose, B.G.; Jackson, M.G. Synthesis of 1λ4,2-diselenol-1-ylium salts. Heteroatom Chem. 1993, 4, 337–342. [Google Scholar] [CrossRef]
- Christie, R.M.; Reid, D.H.J. Studies of heterocyclic compounds. Part XIX. Synthesis of 6,6a-dithia- and 6,6a-diselena-1,2-diazapentalenes. Chem. Soc. Perkin Trans. 1 1976, 2, 228–234. [Google Scholar] [CrossRef]
- Schollmeyer, D.; Detert, H. 3-Methyl-4,5,6,7,8,9-hexahydrocycloocteno-1,2,3-selenadiazolium iodide–trichloromethane (4/1). IUCrData 2017, 2, x170167. [Google Scholar] [CrossRef]
- Schollmeyer, D.; Detert, H. 3-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-3-ium iodide triiodide (3/2/1). IUCrData 2016, 1, x161950. [Google Scholar] [CrossRef]
- Schollmeyer, D.; Detert, H. 3-(2-Ethoxy-2-oxoethyl)-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol-3-ium bromide. IUCrData 2025, 10, x250143. [Google Scholar] [CrossRef]
- Lalezari, I.; Shafiee, A.; Yalpani, M.J. Selenium heterocycles VIII. Pyrolysis of cycloalka-1,2,3-selenadiazoles. Heterocycl. Chem. 1972, 9, 1411–1412. [Google Scholar] [CrossRef]
- Meier, H.; Voigt, E. Bildung und fragmentierung von cycloalkeno-1,2,3-selenadiazolen. Tetrahedron 1972, 28, 187–198. [Google Scholar] [CrossRef]
- Duddeck, H.; Wagner, P.; Müller, D.; Jászberényi, J.C. 77Se and 15N NMR investigation of some cycloalkeno-1,2,3-selenadiazole derivatives. Magn Res. Chem. 1990, 28, 549–552. [Google Scholar] [CrossRef]
- Detert, H.; Meier, H. Chemical and Spectroscopical Properties of Medium Sized trans- and cis-Bicyclo[n.1.0]alk-2-ynes. Liebigs Ann. 1997, 1997, 1565–1570. [Google Scholar] [CrossRef]
- Meier, H.; Zountsas, J.; Zimmer, O. Kernresonanz-Untersuchunghen von 1.2.3-Selenadiazolen/Nuclear Magnetic Resonance of 1,2,3-Selenadiazoles. Z. Naturforsch. B 1981, 36, 1017–1021. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schollmeyer, D.; Detert, H. 2-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-2-ium Iodide. Molbank 2025, 2025, M2082. https://doi.org/10.3390/M2082
Schollmeyer D, Detert H. 2-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-2-ium Iodide. Molbank. 2025; 2025(4):M2082. https://doi.org/10.3390/M2082
Chicago/Turabian StyleSchollmeyer, Dieter, and Heiner Detert. 2025. "2-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-2-ium Iodide" Molbank 2025, no. 4: M2082. https://doi.org/10.3390/M2082
APA StyleSchollmeyer, D., & Detert, H. (2025). 2-Methyl-4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]selenadiazol]-2-ium Iodide. Molbank, 2025(4), M2082. https://doi.org/10.3390/M2082





