Abstract
Imidazothiazoles are important and attractive scaffolds for the design of potential biologically active small molecules. Dialkylenamines are convenient building blocks and are often used as intermediate reagents for the synthesis of various heterocyclic systems such as pyrimidine, pyridine, pyrazole, etc. In the present paper, the simple and effective synthesis of (Z)-6-((dimethylamino)methylene)-2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one (2) is reported. The proposed method, based on the reflux of 2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one with N,N-dimethylformamide dimethyl acetal, leads to an 80% yield of title compound 2. The structure of the synthesized compound 2 was confirmed using 1H, 13C NMR, and LC-MS spectra. The applied protocol demonstrates practical advantages such as the absence of a solvent, a simple work-up, and the possibility of scale-up.
1. Introduction
Imidazothiazoles are a well known class of heterocyclic compounds that are of great importance to medicinal chemistry needs [1,2,3,4]. Among these heterocycles, partially hydrogenated derivatives and analogs are of special interest due to the increasing sp3 atom fraction, which impacts the pharmacodynamic and targets the binding properties of molecules [5]. This group of molecules is mostly known for the drug Levamisole, an active antihelmintic agent widely used for treating parasitic worm infections. Nowadays, derivatives with a wide spectrum of pharmacological properties including agents for the treatment of cancer [6,7,8,9] and other serious diseases [10,11,12,13,14] were identified among imidazothiazoles. On the other hand, imidazothiazoles could be considered bioisosteres of fused heterocyclic systems like thiazolo[3,2-b][1,2,4]triazoles and imidazo[2,1-b][1,3,4]thiadiazoles, which are also significant sources of potential drug-likeness molecules [15,16].
Compounds containing enamine/enaminone and dialkylamino moieties are efficient precursors for the synthesis of different types of heterocyclic systems [17,18]. Moreover, often the introduction of an enamine linker is a strategy for the structural optimization of hit/lead compounds providing favorable molecular properties. 1,1-Dimethoxy-N,N-dimethylmethanamine (DMF-DMA) is one of the most popular and easiest reagents used by synthetic chemists for obtaining the corresponding dimethylamino-methylene derivatives [17,18].
In this paper, we report a straightforward protocol for the synthesis of (Z)-6-((dimethylamino)methylene)-2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one (2), which may be used as a promising reagent in organic and medicinal chemistry. The structure of the compound is fully characterized by NMR spectroscopy and LC-MS spectrometry. The synthesized compound 2 can be used as a convenient and efficient precursor in dimethylamine substitution reactions with indole derivatives, as described in [19], for related heterocyclic systems to obtain aplysinopsin analogs. In addition, compound 2 can be used in condensation reactions with methylene-active compounds for the synthesis of new fused heterocyclic systems, as reported in [20].
2. Results and Discussion
Synthesis of the Title Compound 2
Using DMF-DMA for the synthesis of corresponding enamines in reaction with methylenactive compounds is widely popular, and different conditions for the reaction process were reported depending on the substrate type used in the reaction [21,22,23,24,25,26]. Despite the simplicity of the process, very often, difficulties could be met with targeted product elimination from the reaction mixture, as well as the selectivity of the reaction. Following the strategy and methodology of “green chemistry” [27] for the synthesis of the title compound, we used a solvent-free approach. The synthetic protocol reported in [20] was used, with some optimization for the synthesis of compound 2. The reaction conditions were optimized by using an equimolar amount of DMF-DMA and 2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one (1) and reducing the heating time to 7 h, as seen in Scheme 1. Under the aforementioned reaction conditions, compound 2 was obtained with the best yield (80%). The process was monitored by TLC, and extending the heating time and using excess DMF-DMA led to the formation of by-products that could not be identified and which caused additional difficulties in isolating the main product.
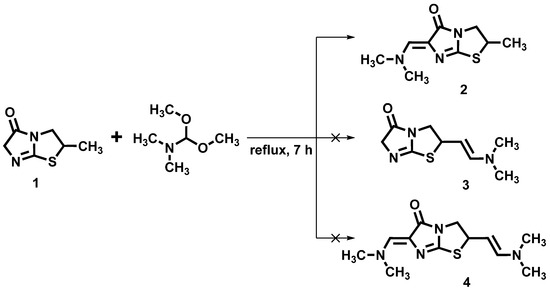
Scheme 1.
Synthesis of title compound 2. Reagents and conditions: 1 (10 mmol), DMF-DMA (10 mmol), reflux 7 h, and yield 80%.
The obtained compound 2 was isolated in crystalline form and further purified by recrystallization, which allowed the avoidance of chromatographic procedures and makes the method convenient for scaling up.
The structure of the synthesized compound 2 was confirmed by 1H, 13C, 2D NMR and LC-MS spectra (copies of spectra are presented in the Supplementary Materials), and key data are highlighted in Figure 1. Considering the chemical properties of DMF-DMA and the presence of several reactive centers in molecule 1, the formation of several hypothetical reaction products is possible, as depicted in Scheme 1. The molecular ion peak observed at the m/z value of 212.0 [M+H]+ in positive ionization mode in the mass spectrum excluded the formation of the theoretically possible compound 4. The presence of a doublet signal in the 1H NMR spectrum at 1.55 ppm corresponds to the methyl group protons at position 2 of the heterocyclic system and excludes the formation of compound 3. Protons of the thiazolidine ring resonate as a multiplet at 4.23–4.31 ppm and a pair of doublet of doublets at 3.47 and 3.97 ppm with corresponding spin–spin coupling constants (2J = 10.8 and 11.0 Hz; 3J = 6.6 and 6.8 Hz) (Figure 1). Signals of methyl groups protons of –N(CH3)2 moiety appear as singlets at 3.13 and 3.42 ppm. Ylidene proton gives a singlet at 6.86 ppm.
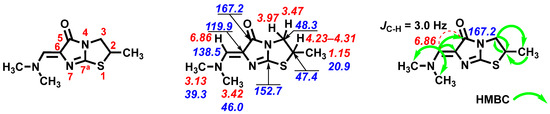
Figure 1.
Atom numbering, chemical shifts, and key interactions based on the relevant HSQC and HMBC spectra of the title compound 2.
In the 13C NMR spectrum, the signals of all carbon atoms are presented (Figure 1). Carbon atoms in the thiazolidine ring give signals at 47.4 (C2) and 48.3 (C3) ppm. Bridgehead carbon at C7a gives a signal at 152.7 ppm. The signals of three methyl group carbons appear at 20.9 (CH3), 39.3 (NCH3), and 46.0 (NCH3) ppm correspondingly. The carbon signal of the carbonyl group (C=O) appears at 167.2 ppm. Ylidene carbon gives a signal at 138.5 ppm and carbon at C6 of the imidazoline ring resonates at 119.9 ppm.
The use of HMBC and HSQC techniques allowed for the confirmation of signal assignments based on 1D NMR techniques (Figure 1). The 3JC-H value for the ylidene proton and the carbonyl group carbon atom (C5) within 3 Hz in the proton-coupled 13C NMR spectrum indicates the Z orientation of the ylidene moiety in the synthesized compound 2.
3. Materials and Methods
Melting points were measured in open capillary tubes on a BÜCHI B-545 melting point apparatus (BÜCHI Labortechnik AG, Flawil, Switzerland) and were uncorrected. The elemental analyses (C, H, and N) were performed using the Perkin-Elmer 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA) and were within ±0.4% of the theoretical values. The 500 MHz 1H and 125 MHz 13C NMR spectra were recorded on a Varian Unity Plus 500 (500 MHz) spectrometer (Varian Inc., Palo Alto, CA, USA). All spectra were recorded at room temperature, except where indicated otherwise, and were referenced internally to solvent reference frequencies. Chemical shifts (δ) are quoted in ppm and coupling constants (J) are reported in Hz. LC-MS spectra were obtained on a Finnigan MAT INCOS-50 (Thermo Finnigan LLC, San Jose, CA, USA). The reaction mixture was monitored by thin-layer chromatography (TLC) using commercial glass-backed TLC plates (Kieselgel 60 F254, Merck KGaA, Darmstadt, Germany). Solvents and reagents that are commercially available were used without further purification. The 2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one 1 was prepared according to the method described in [28].
(Z)-6-((Dimethylamino)methylene)-2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one (2)
A mixture of 2-methyl-2,3-dihydroimidazo[2,1-b]thiazol-5(6H)-one 1 (1.56 g, 10 mmol) and N,N-dimethylformamide dimethyl acetal (1.19 g, 10 mmol) was heated under reflux conditions for 7 h (monitored by TLC). After the completion of the reaction, the mixture was cooled to room temperature and evaporated to obtain a pure white-yellow solid of 2. Formed solid of 2 was recrystallized from methyl tert-butyl ether (MTBE).
Yield 80%, white-yellow crystal powder, mp 142–144 °C (MTBE).
1H NMR (500 MHz, CDCl3, δ): 1.55 (d, 2J = 6.0 Hz, 3H, CH3), 3.13 & 3.42 (2*s, 6H, 2*NCH3), 3.47 (dd, 2J = 11.0, 3J = 6.6 Hz, 1H, CH2), 3.97 (dd, 2J = 10.8, 3J = 6.8 Hz, 1H, CH2), 4.23–4.31 (m, 1H, CH), 6.86 (s, 1H, CH=).
13C NMR (125 MHz, CDCl3, δ): 20.9 (CH3), 39.3 (NCH3), 46.0 (NCH3), 47.4 (C2), 48.3 (C3), 119.9 (C6), 138.5 (CH=), 152.7 (C7a), 167.2 (C=O).
LCMS (Electrospray ionization (ESI+)): m/z 212.0 (100%, [M+H]+).
Anal. calc. for C9H13N3OS: C, 51.41%, H, 6.13%; N, 20.01%; Found: C, 51.60%, H, 6.30%, N, 20.20%.
4. Conclusions
In the present work, we reported an efficient synthetic protocol for the synthesis of a new dimethylenamine-bearing imidazothiazole affording the title compound in good yield without the need for catalysts or extensive purification steps. These advantages make the method attractive for further application in the synthesis of structurally related derivatives. The structure of the compound was characterized and elucidated using NMR spectroscopy and LC-MS spectrometry analysis. The reported compound is a useful building block for the needs of synthetic organic and medicinal chemistry.
Supplementary Materials
Figures S1–S6: 1H NMR, 13C NMR, and LC–MS spectra of compound 2.
Author Contributions
Conceptualization, L.S., N.S. and M.V.; methodology, L.S., S.H., N.S. and M.V.; software, L.S. and S.H.; validation, L.S., S.H. and N.S.; formal analysis, L.S., S.H. and N.S.; investigation, L.S., S.H., N.S. and M.V.; resources, L.S., S.H. and N.S.; data curation, L.S., S.H., N.S. and M.V.; writing—original draft preparation, L.S., S.H. and N.S.; writing—review and editing, L.S., S.H., N.S. and M.V.; visualization, L.S., S.H. and N.S.; supervision, N.S. and M.V.; project administration, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
This research was supported by the Lesya Ukrainka Volyn National University, and the Institute of Organic Chemistry of National Academy of Sciences of Ukraine, which are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fascio, M.L.; Errea, M.I.; D’ACcorso, N.B. Imidazothiazole and Related Heterocyclic Systems. Synthesis, Chemical and Biological Properties. Eur. J. Med. Chem. 2015, 90, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, C.M.A.; Neetha, M.; Aneeja, T.; Anilkumar, G. Synthesis and Applications of Imidazothiazoles: An Overview. ChemistrySelect 2020, 5, 10374–10386. [Google Scholar] [CrossRef]
- Kushwaha, P.; Rashi; Bhardwaj, A.; Khan, D. Synthetic Approaches Toward Imidazo-Fused Heterocycles: A Comprehensive Review. J. Heterocycl. Chem. 2024, 61, 1807–1869. [Google Scholar] [CrossRef]
- Shareef, M.A.; Khan, I.; Babu, B.N.; Kamal, A. A Comprehensive Review on the Therapeutic Versatility of Imidazo [2,1-b]thiazoles. Curr. Med. Chem. 2020, 27, 6864–6887. [Google Scholar] [CrossRef]
- Saliyeva, L.N.; Diachenko, I.V.; Vas’kEvich, R.I.; Slyvka, N.Y.; Vovk, M.V. Imidazothiazoles and their Hydrogenated Analogs: Methods of Synthesis and Biomedical Potential. Chem. Heterocycl. Compd. 2020, 56, 1394–1407. [Google Scholar] [CrossRef]
- Abdel-Maksoud, M.S.; Kim, M.-R.; El-Gamal, M.I.; El-Din, M.M.G.; Tae, J.; Choi, H.S.; Lee, K.-T.; Yoo, K.H.; Oh, C.-H. Design, Synthesis, in Vitro Antiproliferative Evaluation, and Kinase Inhibitory Effects of a New Series of Imidazo[2,1-b]thiazole Derivatives. Eur. J. Med. Chem. 2015, 95, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Morigi, R.; Locatelli, A.; Leoni, A.; Rambaldi, M.; Bortolozzi, R.; Mattiuzzo, E.; Ronca, R.; Maccarinelli, F.; Hamel, E.; Bai, R.; et al. Synthesis, in Vitro and in Vivo Biological Evaluation of Substituted 3-(5-imidazo[2,1-b]thiazolylmethylene)-2-indolinones as New Potent Anticancer Agents. Eur. J. Med. Chem. 2019, 166, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Sbenati, R.M.; Semreen, M.H.; Semreen, A.M.; Shehata, M.K.; Alsaghir, F.M.; El-Gamal, M.I. Evaluation of Imidazo[2,1-b]thiazole-based Anticancer Agents in One Decade (2011–2020): Current Status and Future Prospects. Bioorganic Med. Chem. 2021, 29, 115897. [Google Scholar] [CrossRef]
- Moharram, E.A.; El-Sayed, S.M.; Ghabbour, H.A.; El-Subbagh, H.I. Synthesis, Molecular Modeling Simulations and Anticancer Activity of Some New Imidazo[2,1-b]thiazole Analogues as EGFR/HER2 and DHFR Inhibitors. Bioorganic Chem. 2024, 150, 107538. [Google Scholar] [CrossRef]
- Samala, G.; Devi, P.B.; Saxena, S.; Meda, N.; Yogeeswari, P.; Sriram, D. Design, Synthesis and Biological evaluation of Imidazo[2,1-b]thiazole and Benzo[d]imidazo[2,1-b]thiazole Derivatives as Mycobacterium Tuberculosis Pantothenate Synthetase Inhibitors. Bioorganic Med. Chem. 2016, 24, 1298–1307. [Google Scholar] [CrossRef]
- Saliyeva, L.; Holota, S.; Grozav, A.; Yakovychuk, N.; Litvinchuk, M.; Slyvka, N.; Vovk, M. Synthesis and Evaluation of Antimicrobial and Anti-inflammatory Activity of 6-aryliden-2-methyl-2,3-dihydroimidazo[2,1-b][1,3]thiazoles. Biointerface Res. Appl. Chem. 2021, 12, 292–303. [Google Scholar] [CrossRef]
- Danyliuk, I.; Kovalenko, N.; Tolmachova, V.; Kovtun, O.; Saliyeva, L.; Slyvka, N.; Holota, S.; Kutrov, G.; Tsapko, M.; Vovk, M. Synthesis and Antioxidant Activity Evaluation of Some New 4-thiomethyl functionalised 1,3-thiazoles. Curr. Chem. Lett. 2023, 12, 667–676. [Google Scholar] [CrossRef]
- Slyvka, N.; Saliyeva, L.; Holota, S.; Khyluk, D.; Tkachuk, V.; Vovk, M. Sulfones of Pyridinyloxy-Substituted Imidazo[2,1-b][1,3]thiazines: Synthesis, Anti-Inflammatory Activity Evaluation In Vivo and Docking Studies. Lett. Drug Des. Discov. 2022, 20, 1867–1875. [Google Scholar] [CrossRef]
- Kamboj, P.; Imtiyaz, K.; A Rizvi, M.; Nath, V.; Kumar, V.; Husain, A.; Amir, M. Design, Synthesis, Biological Assessment and Molecular Modeling Studies of Novel imidazothiazole-thiazolidinone Hybrids as Potential Anticancer and Anti-inflammatory Agents. Sci. Rep. 2024, 14, 8547. [Google Scholar] [CrossRef]
- Bhongade, B.A.; Talath, S.; Gadad, R.A.; Gadad, A.K. Biological Activities of imidazo[2,1-b][1,3,4]thiadiazole Derivatives: A Review. J. Saudi Chem. Soc. 2016, 20, S463–S475. [Google Scholar] [CrossRef]
- Mehra, A.; Sangwan, R.; Mehra, A.; Sharma, S.; Wadhwa, P.; Mittal, A. Therapeutic Charisma of Imidazo [2,1-b] [1,3,4]-Thiadiazole Analogues: A Patent Review. Pharm. Pat. Anal. 2023, 12, 177–191. [Google Scholar] [CrossRef]
- Gümüş, M.; Koca, I. Enamines and Dimethylamino Imines as Building Blocks in Heterocyclic Synthesis: Reactions of DMF-DMA Reagent with Different Functional Groups. ChemistrySelect 2020, 5, 12377–12397. [Google Scholar] [CrossRef]
- Bracher, F. Dimethylformamide Acetals and Bredereck’s Reagent as Building Blocks in Natural Products Total Synthesis. Mini-Rev. Org. Chem. 2020, 17, 47–66. [Google Scholar] [CrossRef]
- Jakše, R.; Rečnik, S.; Svete, J.; Golobič, A.; Golič, L.; Stanovnik, B. A Simple Synthesis of Aplysinopsin Analogues by Dimethylamine Substitution in N,N-(dimethylamino)methylidene Derivatives of Five-membered Heterocycles. Tetrahedron 2001, 57, 8395–8403. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Beltagy, D.M.; El-Deeb, I.Y. Microwave-assisted Synthesis of Some New Pyrazolopyridines and Their Antioxidant, Antitumor and Antimicrobial Activities. Eur. J. Med. Chem. 2013, 66, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Nakatani, T.; Tanaka, R.; Okada, M.; Torii, E.; Harayama, T.; Kimachi, T. α-Dimethylaminomethylenation-induced Houben–Hoesch-type Cyclization of Cyanoacetanilides: A Practical Synthesis of 3-formyl-4-hydroxyquinolin-2(1H)-ones. Tetrahedron 2011, 67, 3457–3463. [Google Scholar] [CrossRef]
- Stanovnik, B.; Jakse, R.; Groselj, U.; Sorsak, G.; Svete, J. Synthesis of Thioaplysinopsin Analogs Derived from 5-Dimethylaminomethylidene-2-thioxo-1,3-thiazol-4-ones. HETEROCYCLES 2007, 73, 743–750. [Google Scholar] [CrossRef]
- Fahim, A.M.; Magd, E.E.A.-E. Enhancement of Molecular Imprinted Polymer as Organic Fillers on Bagasse Cellulose Fibers with Biological Evaluation and Computational Calculations. J. Mol. Struct. 2021, 1241, 130660. [Google Scholar] [CrossRef]
- Frolov, A.I.; Ostapchuk, E.N.; Pashenko, A.E.; Chuchvera, Y.O.; Rusanov, E.B.; Volochnyuk, D.M.; Ryabukhin, S.V. Selective α-Methylation of Ketones. J. Org. Chem. 2021, 86, 7333–7346. [Google Scholar] [CrossRef]
- Ciber, L.; Požgan, F.; Brodnik, H.; Štefane, B.; Svete, J.; Grošelj, U. Synthesis and Catalytic Activity of Organocatalysts Based on Enaminone and Benzenediamine Hydrogen Bond Donors. Catalysts 2022, 12, 1132. [Google Scholar] [CrossRef]
- Myshko, A.; Mrug, G.; Kondratyuk, K.; Demydchuk, B.; Bondarenko, S.; Frasinyuk, M. An Expedient Synthesis of Functionalized Pyrazole-Based Aurone Analogs. ChemistrySelect 2023, 8, e202300257. [Google Scholar] [CrossRef]
- Stefanache, A.; Marcinschi, A.; Marin, G.-A.; Mitran, A.-M.; Lungu, I.I.; Miftode, A.M.; Crivoi, F.; Lacatusu, D.; Baican, M.; Cioanca, O.; et al. Green Chemistry Approaches in Pharmaceutical Synthesis: Sustainable Methods for Drug Development. Appliedchem 2025, 5, 13. [Google Scholar] [CrossRef]
- Saliyeva, L.M.; Slyvka, N.Y.; Mel’nyk, D.A.; Rusanov, E.B.; Vas’kevich, R.I.; Vovk, M.V. Synthesis of Spiro[imidazo[2,1-b][1,3]thiazole-6,3′-pyrrolidine] Derivatives. Chem. Heterocycl. Compd. 2018, 54, 130–137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).