1,1-Bis(4-ethylphenyl)-propan-1,2-diol
Abstract
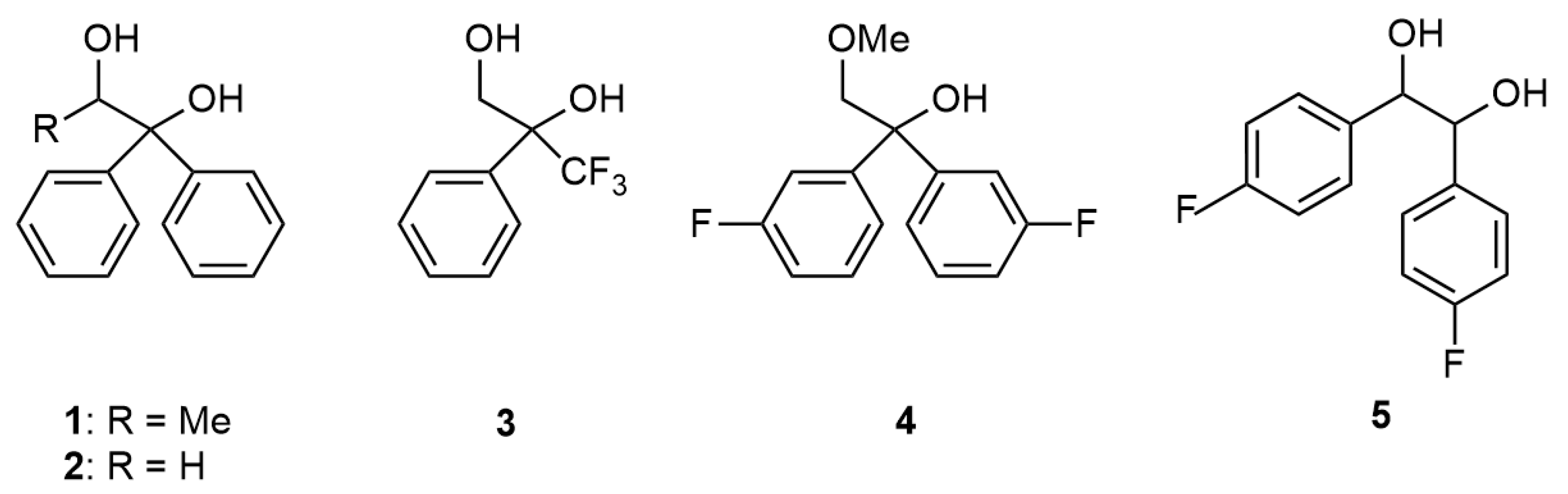
1. Introduction
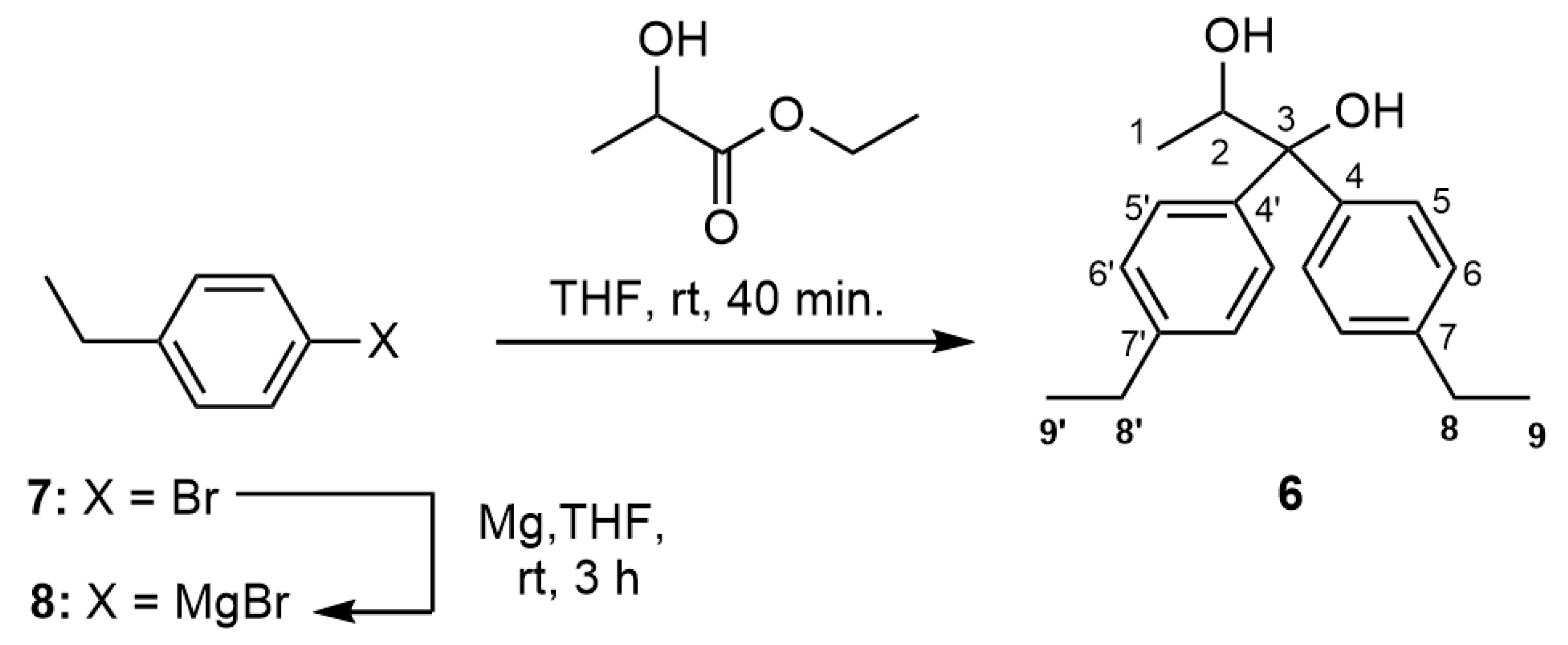
2. Results and Discussion
3. Materials and Methods
3.1. Instrumentation
3.2. 1,1-Bis(4-ethylphenyl)-1,2-propanediol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagherian, G.; Chamjangali, M.A.; Goudarzi, N.; Namazi, N. Selective Spectrophotometric Determination of Periodate Based on Its Reaction with Methylene Green and Its Application to Indirect Determination of Ethylene Glycol and Glycerol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 76, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Kim, J. Assessment of the Potential Risk of 1,2-Hexanediol Using Phytotoxicity and Cytotoxicity Testing. Ecotoxicol. Environ. Saf. 2020, 201, 110796. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.; Zhang, X.; Amini, L.; Dey, P.; Singh, A.K.; Faghani, A.; Schmueck-Henneresse, M.; Haag, R. Functional Surfactants for Molecular Fishing, Capsule Creation, and Single-Cell Gene Expression. Nano-Micro Lett. 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, S.P.; Wu, L.; Wong Chang, M.-A.; Comerford, J.W.; Farmer, T.J.; Schmid, M.; Chang, F.; Li, Z.; Mascal, M. New Bio-Based Monomers: Tuneable Polyester Properties Using Branched Diols from Biomass. Faraday Discuss. 2017, 202, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Sigg, M.; Daniels, R. Investigations on Alkanediols as Alternative Preservatives in a Nonionic Hydrophilic Cream. Pharmaceutics 2020, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gil, S.; Suárez-Pantiga, S.; Pedrosa, M.R.; Sanz, R. Molybdenum-Catalyzed Direct Synthesis of Pyrroles from Nitroarenes with Glycols as Reductants. Adv. Synth. Catal. 2025, 367, e202401170. [Google Scholar] [CrossRef]
- Nalawade, T.; Bhat, K.; Sogi, S.H.P. Bactericidal Activity of Propylene Glycol, Glycerine, Polyethylene Glycol 400, and Polyethylene Glycol 1000 against Selected Microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of 1,2-Glycols as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 147S–168S. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of Propane-1,2-diol (E 1520) as a Food Additive. EFSA J. 2018, 16, e05235. [Google Scholar] [CrossRef] [PubMed]
- Rebiere, F.; Riant, O.; Kagan, H.B. Asymmetric Diels-Alder Reaction Catalysed by Some Chiral Lewis Acids. Tetrahedron Asymmetry 1990, 1, 199–214. [Google Scholar] [CrossRef]
- Mikami, K.; Terada, M.; Nakai, T. Catalytic Asymmetric Glyoxylate-Ene Reaction: A Practical Access to Alpha-Hydroxy Esters in High Enantiomeric Purities. J. Am. Chem. Soc. 1990, 112, 3949–3954. [Google Scholar] [CrossRef]
- Weber, E.; Wimmer, C.; Llamas-Saiz, A.L.; Foces-Foces, C. New Chiral Selectors Derived from Lactic Acid: Cocrystalline and Sorptive Optical Resolutions, and the Crystal Structure of an Inclusion Complex with 3-Methylcyclohexanone. J. Chem. Soc. Chem. Commun. 1992, 733–735. [Google Scholar] [CrossRef]
- Weber, E.; Wimmer, C. Optical Resolution by Crystalline Inclusion Formation Using New Lactic Acid Derived Hosts. Chirality 1993, 5, 315–319. [Google Scholar] [CrossRef]
- Llamas-Saiz, A.L.; Foces-Foces, C.; Weber, E.; Wimmer, C. Chiral Crystalline Hosts Derived from Lactic Acid. X-ray Crystal Structures of Optically Resolved and Racemic Host Compound 1,1-Diphenyl-1,2-propanediol and a 2:1 Complex between Optically Resolved Host and 3-Picoline. Supramol. Chem. 1993, 2, 215–223. [Google Scholar] [CrossRef]
- Reinbold, J.; Buhlmann, K.; Cammann, K.; Wierig, A.; Wimmer, C.; Weber, E. Inclusion of Organic Vapours by Crys-talline Hosts. Chemical-Sensitive Coatings for Sensor Applications. Sens. Actuators B Chem. 1994, 18, 77–81. [Google Scholar] [CrossRef]
- Castillo-Ramirez, J.; Echevarría, I.; Santiago, J.; Pérez-Torres, M.; Rivera-Claudio, M. Synthesis and Characterization of Ferrocene Acetals and Evaluation of Their Antineoplastic Properties by Using Breast Cancer Cell Lines In Vitro. Synthesis 2013, 45, 1853–1856. [Google Scholar] [CrossRef]
- Jomhori, M.; Mosaddeghi, H. Molecular Modeling of Natural and Synthesized Inhibitors against SARS-CoV-2 Spike Glycoprotein. Res. Biomed. Eng. 2022, 38, 71–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashida, I.; Uktamova, M.; Tirkasheva, S.; Torikai, K. 1,1-Bis(4-ethylphenyl)-propan-1,2-diol. Molbank 2025, 2025, M2076. https://doi.org/10.3390/M2076
Hayashida I, Uktamova M, Tirkasheva S, Torikai K. 1,1-Bis(4-ethylphenyl)-propan-1,2-diol. Molbank. 2025; 2025(4):M2076. https://doi.org/10.3390/M2076
Chicago/Turabian StyleHayashida, Ichika, Malokhat Uktamova, Sarvinoz Tirkasheva, and Kohei Torikai. 2025. "1,1-Bis(4-ethylphenyl)-propan-1,2-diol" Molbank 2025, no. 4: M2076. https://doi.org/10.3390/M2076
APA StyleHayashida, I., Uktamova, M., Tirkasheva, S., & Torikai, K. (2025). 1,1-Bis(4-ethylphenyl)-propan-1,2-diol. Molbank, 2025(4), M2076. https://doi.org/10.3390/M2076






