3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid
Abstract
1. Introduction
2. Results
2.1. Synthesis and Spectroscopy
2.2. Crystal Structure Description
2.3. Structure Comparison
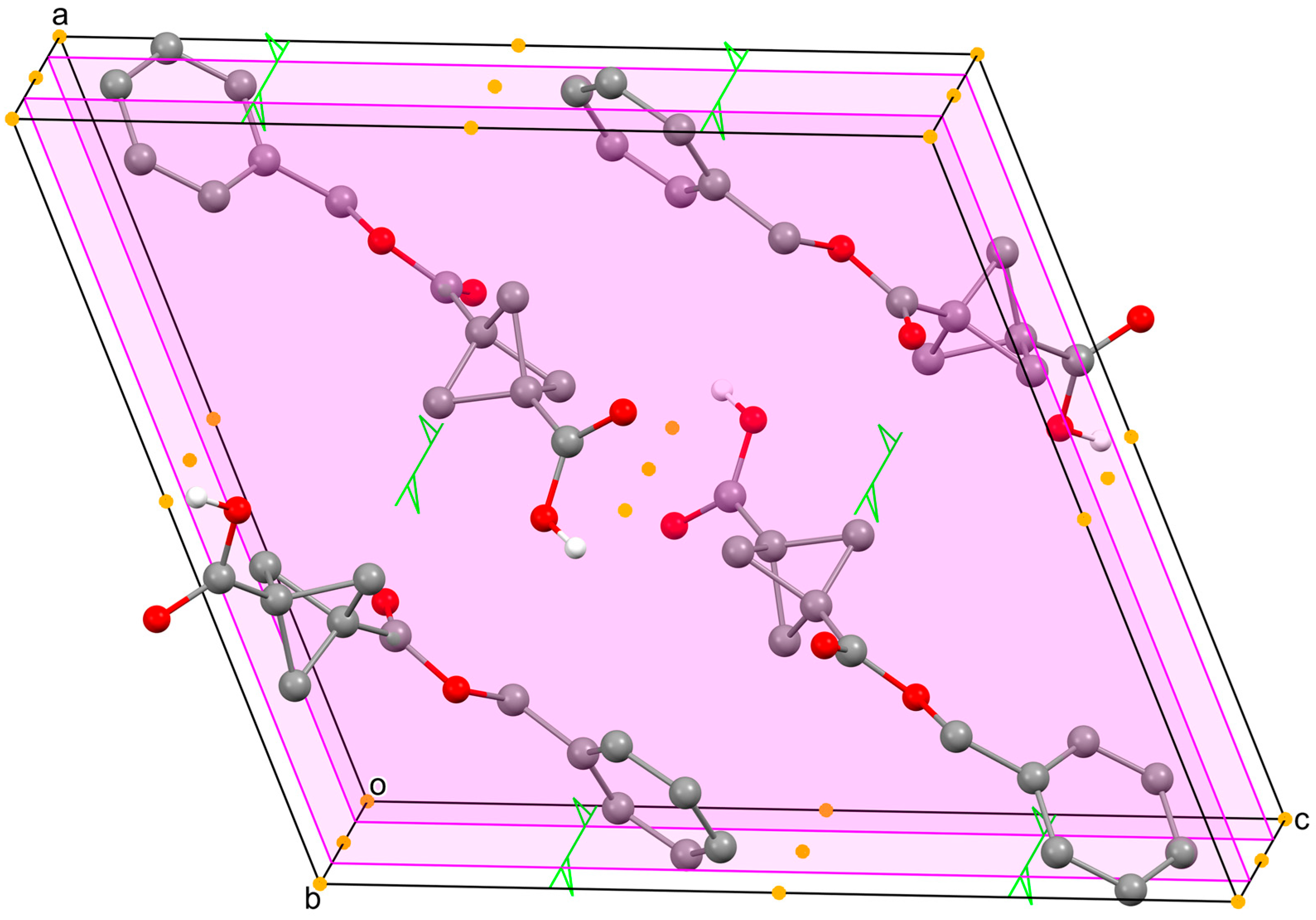
2.4. Lattice Structure
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. General Materials and Procedures
3.1.2. Dibenzyl Bicyclo[1.1.1]pentane-1,3-dicarboxylate (1)
3.1.3. 3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic acid (2)
3.2. Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Hydrogen acceptor |
| BA | Benzoic acid |
| BCP | Bicyclo[1.1.1]pentane; Bicyclo[1.1.1]pentyl |
| CSD | Cambridge structural database |
| D | Hydrogen donor |
| Dcalc | Calculated density from X-ray data |
| DCM | Dichloromethane |
| DMF | Dimethylformamide |
| EtOAc | Ethyl acetate |
| NMR | Nuclear magnetic resonance |
| SC-XRD | Single-crystal X-ray diffraction |
| THF | Tetrahydrofuran |
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K. Saturated bioisosteres of benzene: Where to go next? Org. Biomol. Chem. 2019, 17, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Zhang, H.; Guo, L.; Cheng, M.; Doty, A.C.; Ferguson, H.; Fradera, X.; Lesburg, C.A.; McGowan, M.A.; Miller, J.R.; et al. Discovery of Potent and Orally Available Bicyclo[1.1.1]pentane-Derived Indoleamine-2,3-dioxygenase 1 (IDO1) Inhibitors. ACS Med. Chem. Lett. 2020, 11, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Typical interatomic distances: Organic compounds. In International Tables for Crystallography Volume C: Mathematical, Physical and Chemical Tables; Springer: Dordrecht, The Netherlands, 2006; pp. 790–811. [Google Scholar]
- Levin, M.D.; Kaszynski, P.; Michl, J. Bicyclo[1.1.1]pentanes, [n]Staffanes, [1.1.1]Propellanes, and Tricyclo[2.1.0.02,5]pentanes. Chem. Rev. 2000, 100, 169–234. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.C.; Shankland, N.; Florence, A.J. A single-crystal neutron diffraction study of the temperature dependence of hydrogen-atom disorder in benzoic acid dimers. J. Chem. Soc. Faraday Trans. 1996, 92, 5051–5057. [Google Scholar] [CrossRef]
- Allen, F.; Bruno, I. Bond lengths in organic and metal-organic compounds revisited: X—H bond lengths from neutron diffraction data. Acta Crystallogr. B 2010, 66, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Kleemiss, F.; Dolomanov, O.V.; Bodensteiner, M.; Peyerimhoff, N.; Midgley, L.; Bourhis, L.J.; Genoni, A.; Malaspina, L.A.; Jayatilaka, D.; Spencer, J.L.; et al. Accurate crystal structures and chemical properties from NoSpherA2. Chem. Sci. 2020, 12, 1675–1692. [Google Scholar] [CrossRef] [PubMed]
- Fillaux, F.; Cousson, A. A neutron diffraction study of the crystal of benzoic acid from 6 to 293K and a macroscopic-scale quantum theory of the lattice of hydrogen-bonded dimers. Chem. Phys. 2016, 479, 26–35. [Google Scholar] [CrossRef]
- Kurzydłowski, D. Potential energy barrier for proton transfer in compressed benzoic acid. RSC Adv. 2022, 12, 11436–11441. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72 Pt 2, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.; Locke, G.M.; Flanagan, K.J.; Beh, M.H.R.; Thompson, A.; Senge, M.O. Bridging and Conformational Control of Porphyrin Units through Non-Traditional Rigid Scaffolds. Chem. Eur. J. 2020, 26, 2405–2416. [Google Scholar] [CrossRef]
- Kanazawa, J.; Maeda, K.; Uchiyama, M. Radical Multicomponent Carboamination of [1.1.1]Propellane. J. Am. Chem. Soc. 2017, 139, 17791–17794. [Google Scholar] [CrossRef] [PubMed]
- Yin, D. CCDC 2350394 Experimental Crystal Structure Determination. 2024. [CrossRef]
- Ripenko, V.; Sham, V.; Levchenko, V.; Holovchuk, S.; Vysochyn, D.; Klymov, I.; Kyslyi, D.; Veselovych, S.; Zhersh, S.; Dmytriv, Y.; et al. Light-enabled scalable synthesis of bicyclo[1.1.1]pentane halides and their functionalizations. Nat. Synth. 2024, 3, 1538–1549. [Google Scholar] [CrossRef]
- Luger, P.; Weber, M.; Szeimies, G.; Pätzel, M. 3-(tert-Butyl-oxy-carbonyl-amino)-bicyclo-[1.1.1]-pentane-carboxyl-ic acid at 293 K. Acta Crystallogr. C 2000, 56, 1170–1172. [Google Scholar] [CrossRef]
- Ripenko, V.; Vysochyn, D.; Klymov, I.; Zhersh, S.; Mykhailiuk, P.K. Large-Scale Synthesis and Modifications of Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acid (BCP). J. Am. Chem. Soc. 2021, 86, 14061–14068. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Yarnton, UK, 2014. [Google Scholar]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallograp. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. A 2015, 71 Pt 1, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]

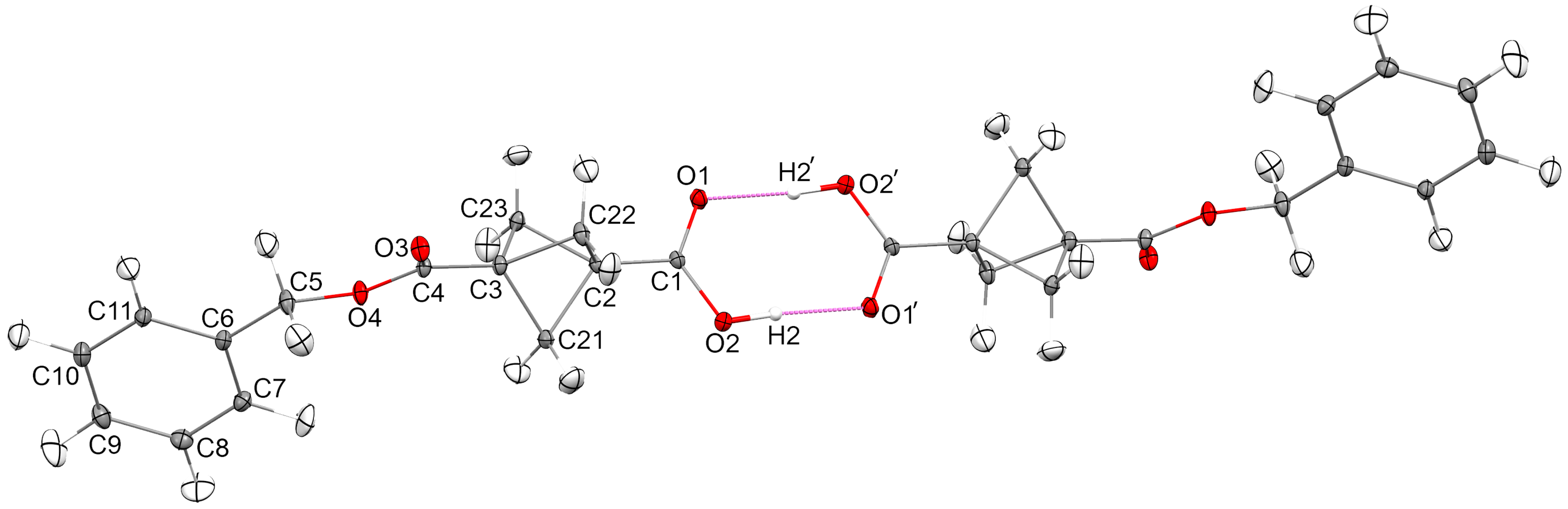
| Bond | Length (Å) | Csp3 (Å) a | Csp2 (Å) a |
|---|---|---|---|
| C–C bonds in the BCP moiety | |||
| C2–C21 | 1.5485(9) | ||
| C2–C22 | 1.5655(9) | ||
| C2–C23 | 1.5504(9) | ||
| C3–C21 | 1.5623(9) | ||
| C3–C22 | 1.5448(9) | ||
| C3–C23 | 1.5626(9) | ||
| Average a | 1.557 ± 0.008 | 1.530 | |
| Bridgehead to substituent | |||
| C1–C2 | 1.4866(9) | 1.502 | 1.476 |
| C3–C4 | 1.4888(9) | 1.497 | 1.489 |
| Bond Length (Å) | Lower Quartile (Å) | Upper Quartile (Å) | ΔCO (Å) | |
|---|---|---|---|---|
| Carbonyl (C=O) | ||||
| C1-O1 | 1.2385(9) | – | – | 0.062 |
| C4-O3 | 1.2083(9) | |||
| C–C(=O)–OH | 1.214 a | 1.203 a | 1.224 a | |
| Bond to the hydroxyl group (C–O) | ||||
| C1-O2 | 1.3001(8) | – | – | 0.132 |
| C4-O4 | 1.3398(8) | |||
| C–C(=O)–OH | 1.308 a | 1.308 a | 1.298 a | |
| D H A | d(D–H) (Å) | d(H–A) (Å) | d(D–A) (Å) | D–H–A (°) |
|---|---|---|---|---|
| O2 H2 O1 | 0.9900(19) | 1.650(3) | 2.6376(7) | 174.5(14) |
| O1 H1a O2′ | 0.990(2) | 1.654(10) | 2.6376(7) | 172(7) |
| Refcode | Angle 1 (°) a | Angle 2 (°) a | Angle 3 (°) a | C2···C3 (Å) b | Ref. |
|---|---|---|---|---|---|
| DIGGOT | 73.8(2) | 73.3(1) | 73.8(2) | 1.859(2) | [12] |
| SUJKIU | 73.8(1) | 73.8(1) | 73.5(1) | 1.866(2) | [12] |
| SUJKOA | 73.8(1) | 73.4(1) | 73.1(1) | 1.861(2) | [12] |
| WERXOJ | 73.78(7) | 73.20(7) | 73.00(7) | 1.853(1) | [13] |
| YOMPAV | 73.9(1) | 73.7(1) | 73.7(1) | 1.868(2) | [14] |
| DUFWOU | 74.5(2) | 73.8(2) | 73.8(2) | 1.871(3) | [15] |
| WOCXIV01 | 74.00(4) | 73.81(4) | 73.49(4) | 1.868(9) | [16] |
| Average | 73.9 ± 0.2 | 73.6 ± 0.2 | 73.4 ± 0.3 | 1.864 ± 0.006 | |
| 2 | 73.60(5) | 73.58(5) | 73.52(5) | 1.863(1) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toporkov, D.D.; Nelson, S.K.; Hamel, J.-D.; Boeré, R.T. 3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid. Molbank 2025, 2025, M2075. https://doi.org/10.3390/M2075
Toporkov DD, Nelson SK, Hamel J-D, Boeré RT. 3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid. Molbank. 2025; 2025(4):M2075. https://doi.org/10.3390/M2075
Chicago/Turabian StyleToporkov, Dennis D., Stacie K. Nelson, Jean-Denys Hamel, and René T. Boeré. 2025. "3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid" Molbank 2025, no. 4: M2075. https://doi.org/10.3390/M2075
APA StyleToporkov, D. D., Nelson, S. K., Hamel, J.-D., & Boeré, R. T. (2025). 3-((Benzyloxy)carbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid. Molbank, 2025(4), M2075. https://doi.org/10.3390/M2075







