3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one
Abstract
1. Introduction
2. Results and Discussion
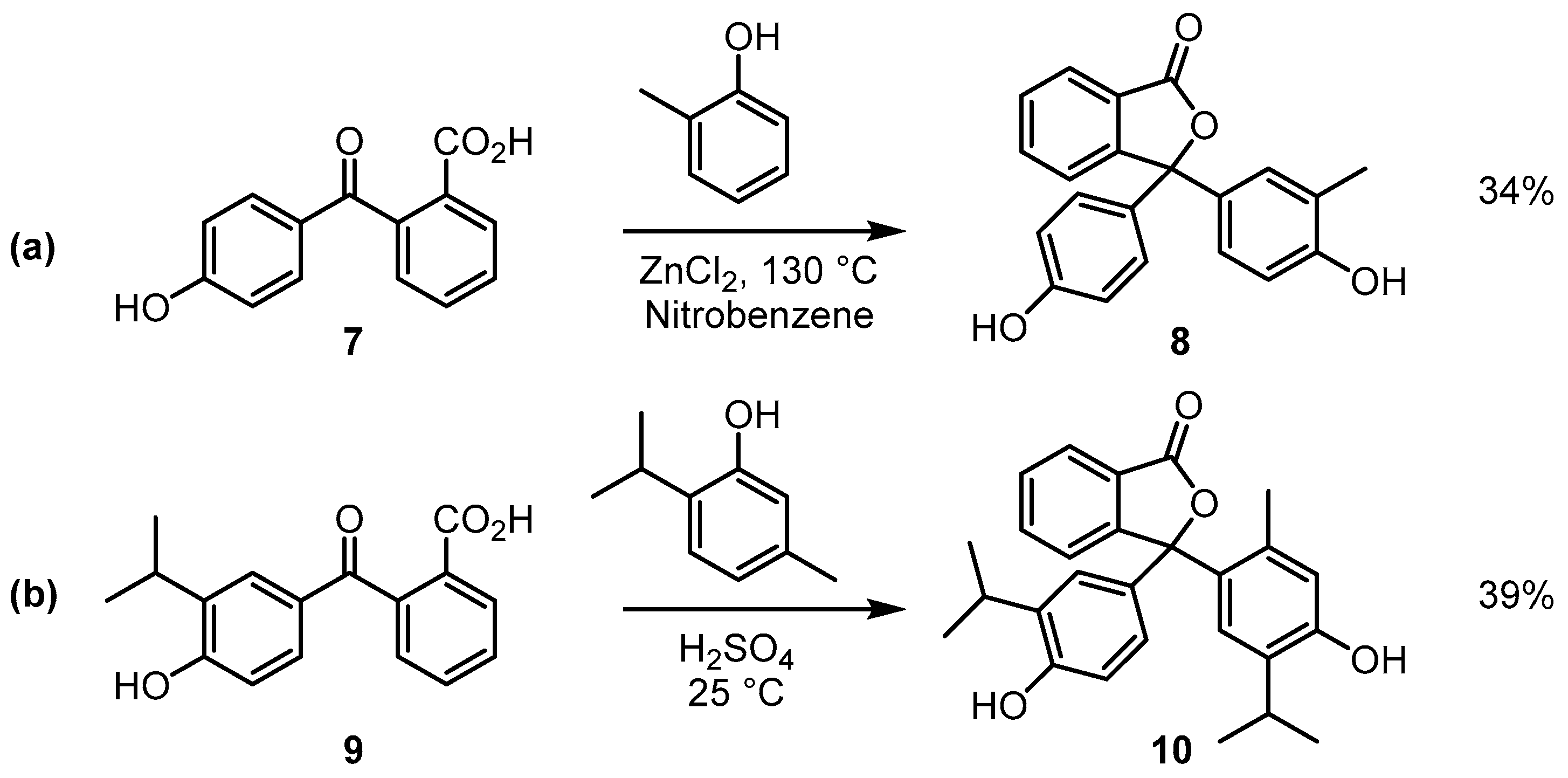
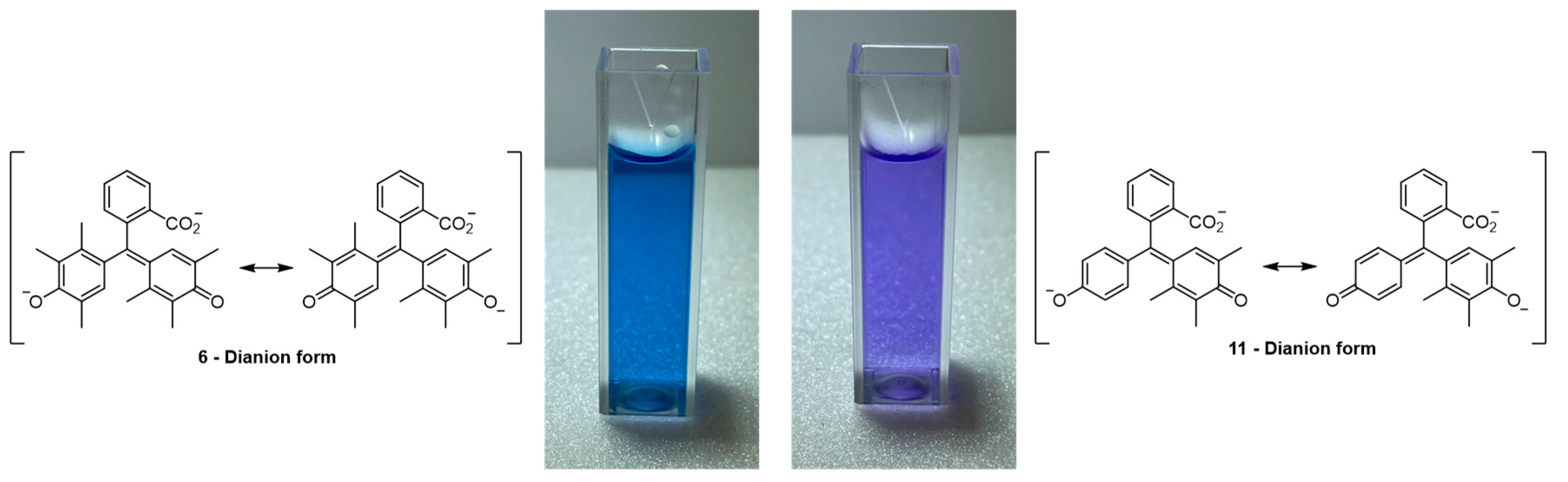
2.1. Synthesis and Spectroscopy
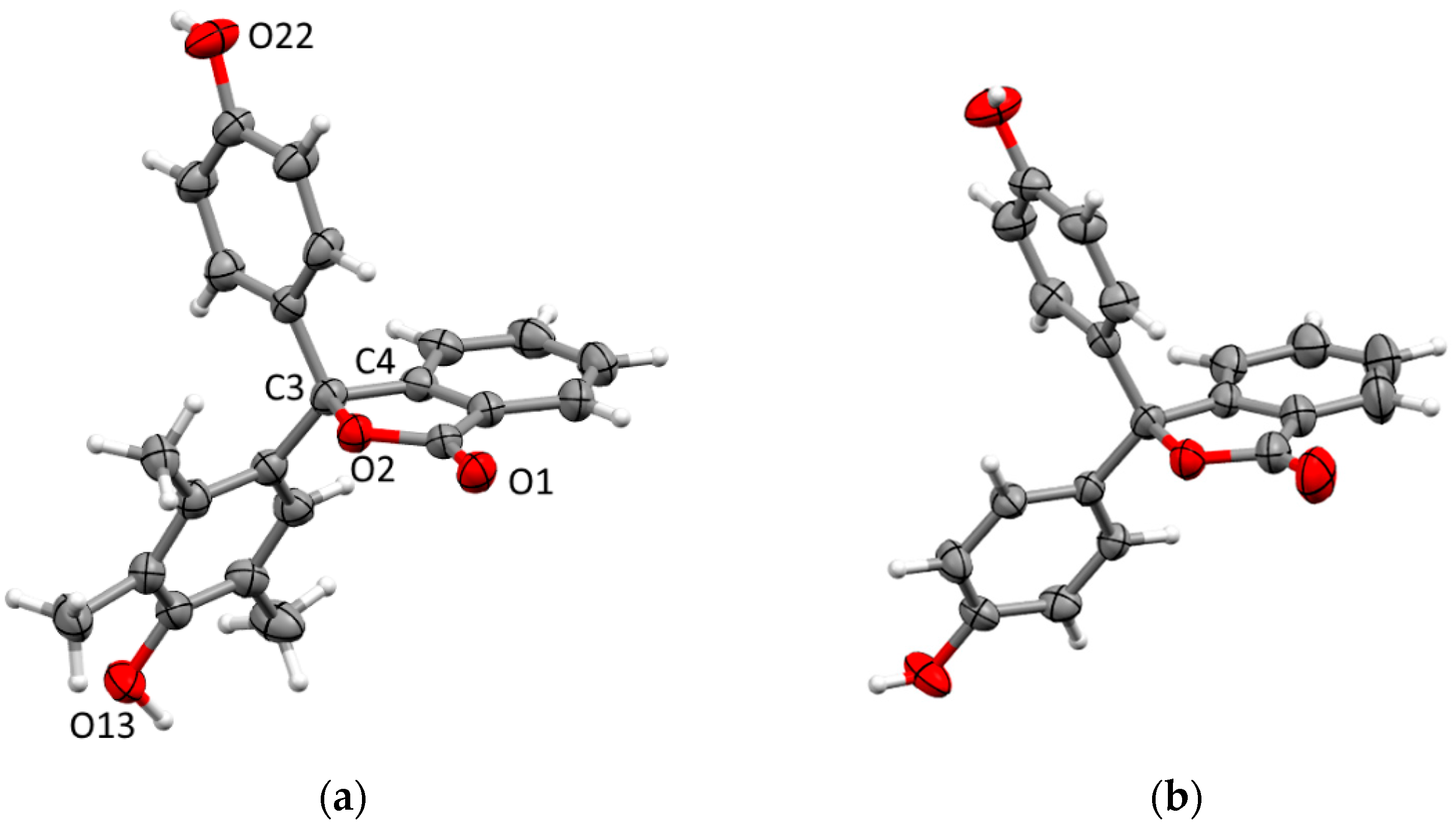
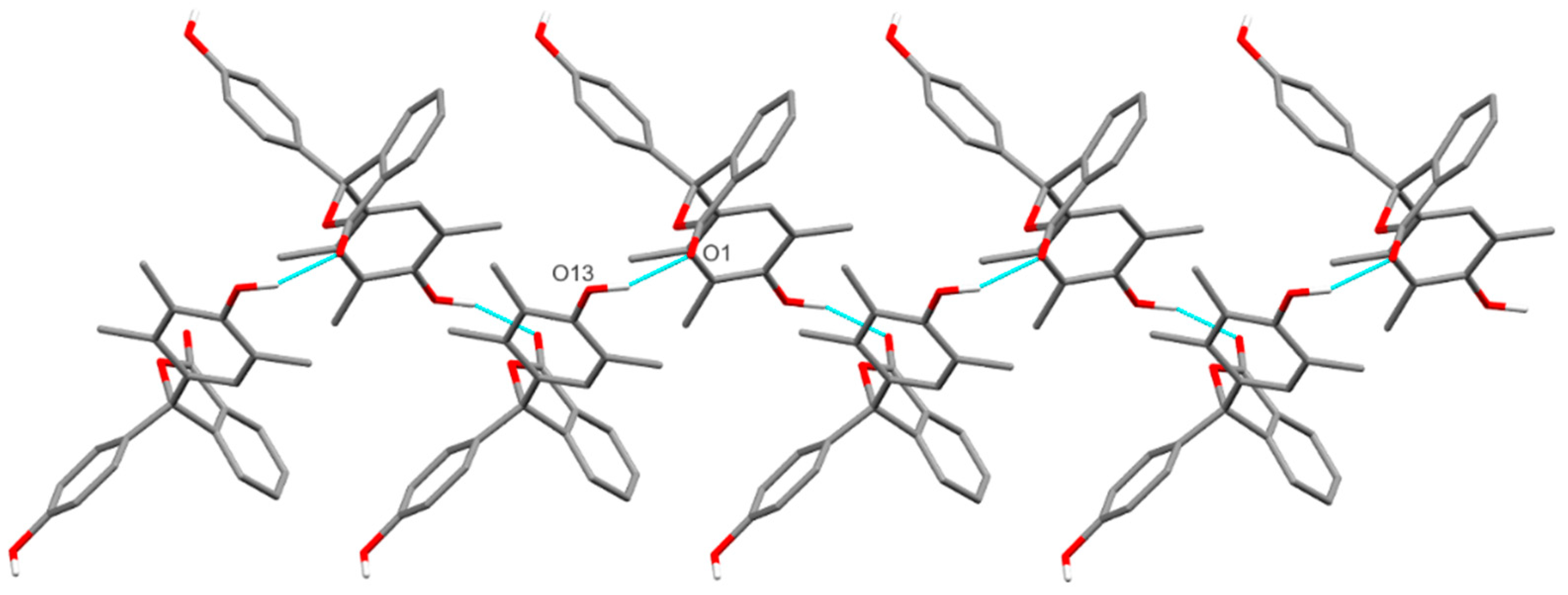
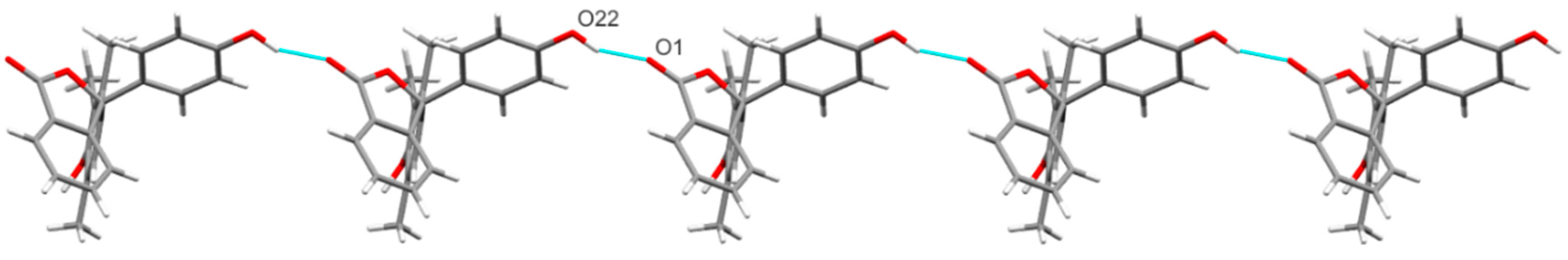
2.2. The Crystal Structure of 11
3. Experimental Section
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabnis, R.W. A facile synthesis of phthalein dyes. Tetrahedron Lett. 2009, 50, 6261–6263. [Google Scholar] [CrossRef]
- Sabnis, R.W. Developments in the chemistry and applications of phthalein dyes. Part 1: Industrial applications. Color. Technol. 2018, 134, 187–205. [Google Scholar] [CrossRef]
- Baeyer, A. Ueber die Phenolfarbstoffe. Ber. Dtsch. Chem. Ges. 1871, 4, 658–665. [Google Scholar] [CrossRef]
- Moreno, V.F.; dos Santos, G.C.; da Costa, G.M.G.; Gomes, M.H.A.; da Silva-Filho, L.C. NbCl5 Promoted the efficient synthesis of phthalein derivatives: Optical characterization and solvatochromic effect. J. Heterocycl. Chem. 2019, 56, 2811–2821. [Google Scholar] [CrossRef]
- Wittke, G. Reactions of Phenolphthalein at Various pH Values. J. Chem. Educ. 1983, 60, 239–240. [Google Scholar] [CrossRef]
- Lund, H. CCIV.—The constitution of phenolphthalein. Part 1. Preparation of some compounds of the phthalein type. J. Chem. Soc. 1928, 1569–1575. [Google Scholar] [CrossRef]
- Nekhoroshev, S.V.; Nekhoroshev, V.P.; Poleshchuk, O.K.; Yarkova, A.G.; Nekhorosheva, A.V.; Gasparyan, A.K. New chemical markers based on phthaleins. Russ. J. Appl. Chem. 2015, 88, 711–718. [Google Scholar] [CrossRef]
- Rumiński, J.K.; Przewoska, K.D. Synthesis and Reactivity of 2-Aroylbenzoic Acids, II. 2-(4-Hydroxy-3-isopropylbenzoyl)benzoic Acid. Chem. Ber. 1982, 115, 3436–3443. [Google Scholar] [CrossRef]
- Hubacher, M.H. The Preparation of 2-(Hydroxybenzoyl)-benzoic Acid. J. Am. Chem. Soc. 1946, 68, 718–719. [Google Scholar] [CrossRef]
- Allen, F.H.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Typical interatomic distances: Organic compounds. In International Tables for Crystallography, 3rd ed.; Allen, F.H., Watson, D.G., Brammer, L., Orpen, A.G., Taylor, R., Eds.; John Wiley & Sons: New York, NY, USA, 2006; Volume 3, pp. 790–811. [Google Scholar]
- Sugira, H.; Kato, T.; Senda, H.; Kunimoto, K.K.; Kuwae, A.; Hanai, K. Crystal Structure of Phenolphthalein. Anal. Sci. 1999, 15, 611–612. [Google Scholar] [CrossRef]
- Fitzgerald, L.J.; Gerkin, R.E. Phenolphthalein and 3′,3′′-Dinitrophenolphthalein. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 535–539. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, v1.171.43.109a & 129a; Rigaku Oxford Diffraction, Rigaku Corporation: Tokyo, Japan, 2024.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
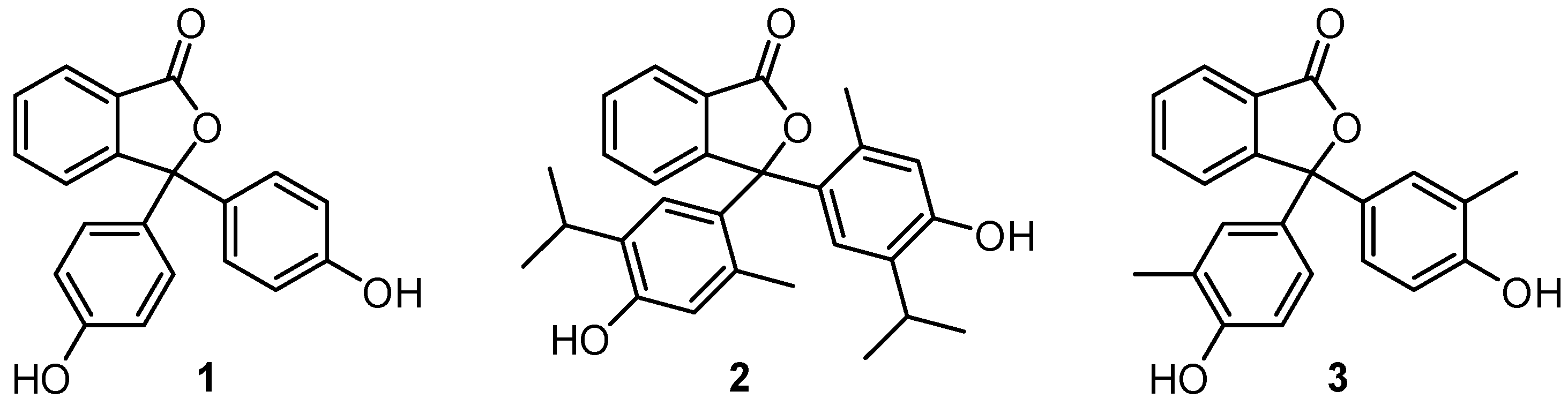

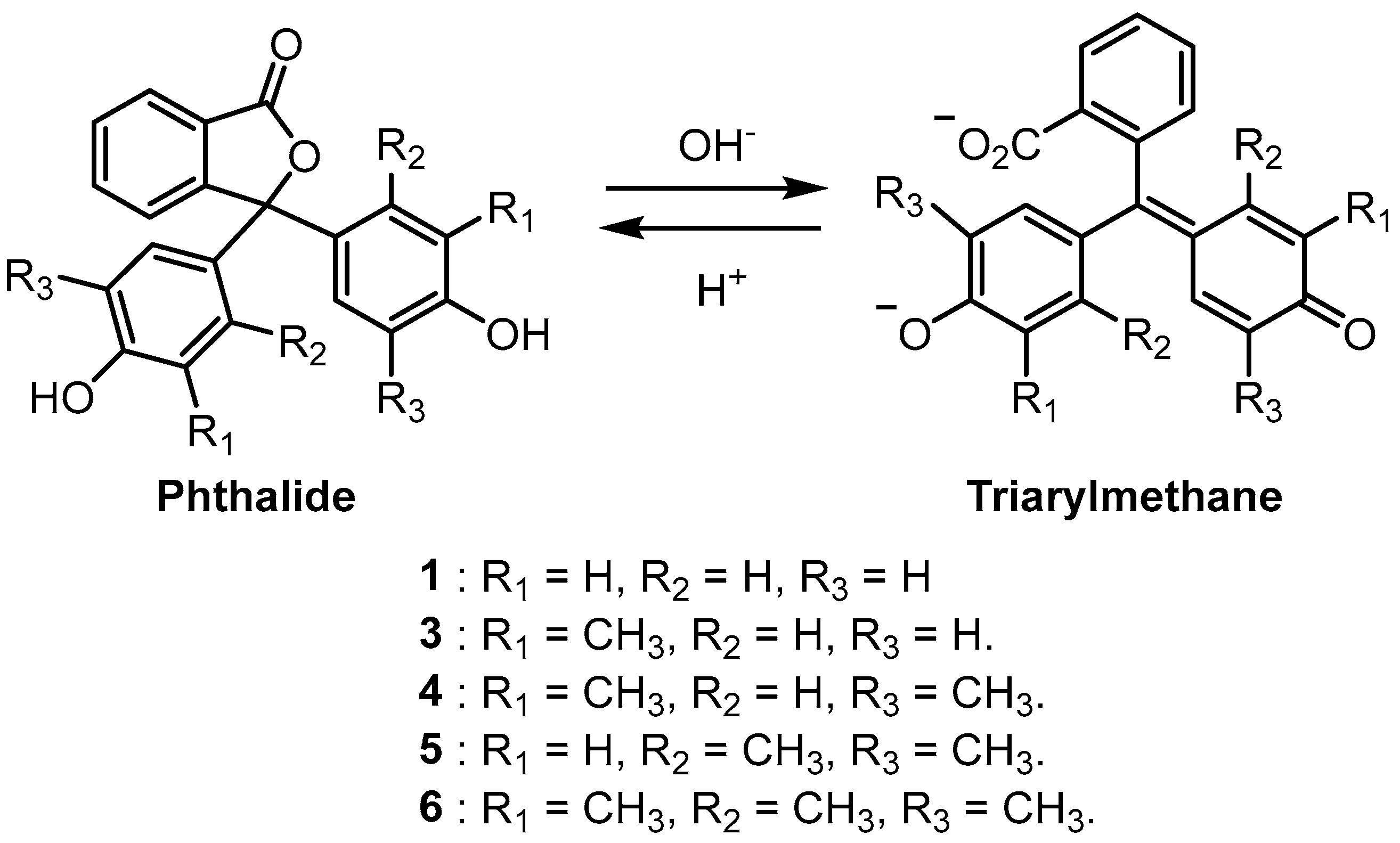

| pH Indicator | R1 | R2 | R3 | Colour of Basic Solution |
|---|---|---|---|---|
| Phenolphthalein (1) | H | H | H | Pink |
| Cresolphthalein (3) | CH3 | H | H | Magenta |
| 2,6-Dimethylphenolphthalein (4) | CH3 | H | CH3 | Violet |
| 2,5-Dimethylphenolphthalein (5) | H | CH3 | CH3 | Blue |
| 2,3,6-Trimethylphenolphthalein (6) | CH3 | CH3 | CH3 | Teal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmers, B.A.; Cordes, D.B.; McKay, A.P.; Patterson, I.L.J.; Pearson, R.J.; Vladymyrova, N.; Smellie, I.A. 3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one. Molbank 2025, 2025, M2067. https://doi.org/10.3390/M2067
Chalmers BA, Cordes DB, McKay AP, Patterson ILJ, Pearson RJ, Vladymyrova N, Smellie IA. 3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one. Molbank. 2025; 2025(4):M2067. https://doi.org/10.3390/M2067
Chicago/Turabian StyleChalmers, Brian A., David B. Cordes, Aidan P. McKay, Iain L. J. Patterson, Russell J. Pearson, Nadiia Vladymyrova, and Iain A. Smellie. 2025. "3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one" Molbank 2025, no. 4: M2067. https://doi.org/10.3390/M2067
APA StyleChalmers, B. A., Cordes, D. B., McKay, A. P., Patterson, I. L. J., Pearson, R. J., Vladymyrova, N., & Smellie, I. A. (2025). 3-(4-Hydroxy-2,3,5-trimethylphenyl)-3-(4-hydroxyphenyl)isobenzofuran-1(3H)-one. Molbank, 2025(4), M2067. https://doi.org/10.3390/M2067






