Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene
Abstract
1. Introduction
2. Results
2.1. Structural Commentary
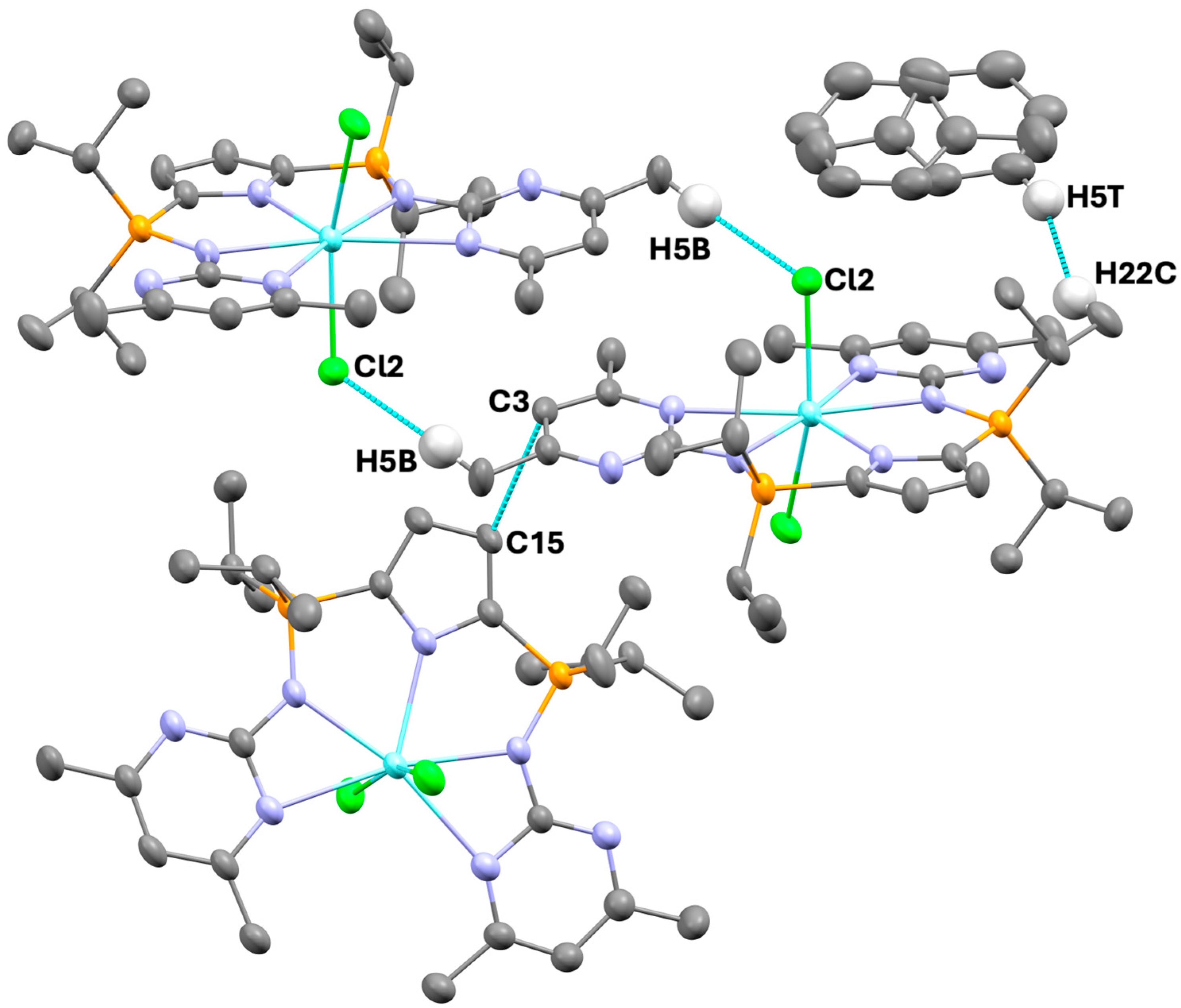
2.2. Supramolecular Features
3. Materials and Methods
3.1. General Methods
3.2. Synthesis
3.3. Crystallization and Refinement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piers, W.E.; Emslie, D.J.H. Non-cyclopentadienyl ancillaries in organogroup 3 metal chemistry: A fine balance in ligand design. Coord. Chem. Rev. 2002, 233, 131–155. [Google Scholar] [CrossRef]
- Zamora, M.T.; Johnson, K.R.D.; Hanninen, M.M.; Hayes, P.G. Differences in the cyclometallation reactivity of bisphosphinimine-supported organo-rare earth complexes. Dalton Trans. 2014, 43, 10739–10750. [Google Scholar] [CrossRef]
- Johnson, K.R.D.; Hayes, P.G. Synthesis and reactivity of dialkyl lutetium complexes supported by a novel bis(phosphinimine)carbazole pincer ligand. Organometallics 2009, 28, 6352–6361. [Google Scholar] [CrossRef]
- Johnson, K.R.D.; Hannon, M.A.; Ritch, J.S.; Hayes, P.G. Thermally stable rare earth diakyl complexes supported by a novel bis(phosphinimine)pyrrole ligand. Dalton Trans. 2012, 41, 7873–7875. [Google Scholar] [CrossRef]
- Knott, J.P.; Hanninen, M.M.; Rautianinen, J.K.; Tuononen, H.M.; Hayes, P.G. Insights into the decomposition pathway of a lutetium alkylamido complex via intramolecular C–H bond activation. J. Organomet. Chem. 2017, 845, 135–143. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Gordon, J.C. Complexes containing multiple bonding interactions between lanthanoid elements and main-group fragments. RCS Adv. 2013, 3, 6682–6692. [Google Scholar] [CrossRef]
- Lu, E.; Li, Y.; Chen, Y. A scandium terminal imido complex: Synthesis, structure and DFT studies. Chem. Commun. 2010, 46, 4469–4471. [Google Scholar] [CrossRef]
- Lu, E.; Chu, J.; Chen, Y. Scandium Terminal Imido Chemistry. Acc. Chem. Res. 2018, 51, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, D.; Zhu, M.; Wei, J.; Zhang, W.X. Synergism between cyclopentadienyl and amidinate ligands affording anionic scandium terminal imido complexes. Inorg. Chem. Front. 2025, 12, 3791–3799. [Google Scholar] [CrossRef]
- Lu, E.; Chu, J.; Chen, Y.; Borzov, M.V.; Li, G. Scandium terminal imido complex induced C-H bond selenation and formation of an Sc-Se bond. Chem. Commun. 2011, 47, 743–745. [Google Scholar] [CrossRef]
- Schädle, D.; Meermann-Zimmermann, M.; Schädle, C.; Maichle-Mössmer, C.; Anwander, R. Rare-Earth Metal Complexes with Terminal Imido Ligands. Eur. J. Inorg. Chem. 2015, 2015, 1334–1339. [Google Scholar] [CrossRef]
- Knott, J.P.; Hsiang, S.J.; Hayes, P.G. Alkylamido lutetium complexes as prospective lutetium imido precursors: Synthesis, characterization and ligand design. Dalton Trans. 2025, 54, 6261–6273. [Google Scholar] [CrossRef]
- Roitershtein, D.; Domingos, Â.; Pereira, L.C.J.; Ascenso, J.R.; Marques, N. Coordination of 2,2‘-Bipyridyl and 1,10-Phenanthroline to Yttrium and Lanthanum Complexes Based on a Scorpionate Ligand. Inorg. Chem. 2003, 42, 7666–7673. [Google Scholar] [CrossRef]
- Caballo, J.; Mena, M.; Pérez-Redondo, A.; Yélamos, C. Cyclopentadienyl yttrium complexes with the [{Ti(η5-C5Me5)(μ-NH)}3(μ3-N)] metalloligand. J. Organomet. Chem. 2019, 896, 139–145. [Google Scholar] [CrossRef]
- Caballo, J.; Carbó, J.J.; Mena, M.; Pérez-Redondo, A.; Poblet, J.M.; Yélamos, C. Redox-Active Behavior of the [{Ti(η5-C5Me5)(μ-NH)}3(μ3-N)] Metalloligand. Inorg. Chem. 2013, 52, 6103–6109. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.N.Y.; Humbert-Droz, M.; Dutronc, T.; Guénée, L.; Besnard, C.; Piguet, C. A Polyaromatic Terdentate Binding Unit with Fused 5,6-Membered Chelates for Complexing s-, p-, d-, and f-Block Cations. Inorg. Chem. 2013, 52, 5570–5580. [Google Scholar] [CrossRef]
- Evans, W.J.; Shreeve, J.L.; Boyle, T.J.; Ziller, J.W. Coordination chemistry of N-methylimidazole with yttrium and cerium. J. Coord. Chem. 1995, 34, 229–239. [Google Scholar] [CrossRef]
- Petersen, J.B.; Ding, Y.-S.; Gupta, S.; Borah, A.; McInnes, E.J.L.; Zheng, Y.-Z.; Murugavel, R.; Winpenny, R.E.P. Electron Paramagnetic Resonance Spectra of Pentagonal Bipyramidal Gadolinium Complexes. Inorg. Chem. 2023, 62, 8435–8441. [Google Scholar] [CrossRef]
- Bai, S.D.; Tong, H.B.; Guo, J.P.; Zhou, M.-S.; Liu, D.S.; Yuan, S.F. Diverse coordination behaviors of the silyl-linked bis(amidinate_ ligand [SiMe2{NC(Ph)N(Ph)}2]− to zirconium center. Polyhedron 2010, 29, 262–269. [Google Scholar] [CrossRef]
- Sobota, P.; Utko, J.; Szafert, S. Ionization of YCI3 in Tetrahydrofuran. Crystal Structures of the [trans-YCl2(THF)5][trans-YCl4(THF)2] Salt and Polymeric [YCl3·2THF]∞ Compounds. Inorg. Chem. 1994, 33, 5203–5206. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the moderncomputing environment–Olex2 dissected. Acta Cryst. 2015, A71, 59–75. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]

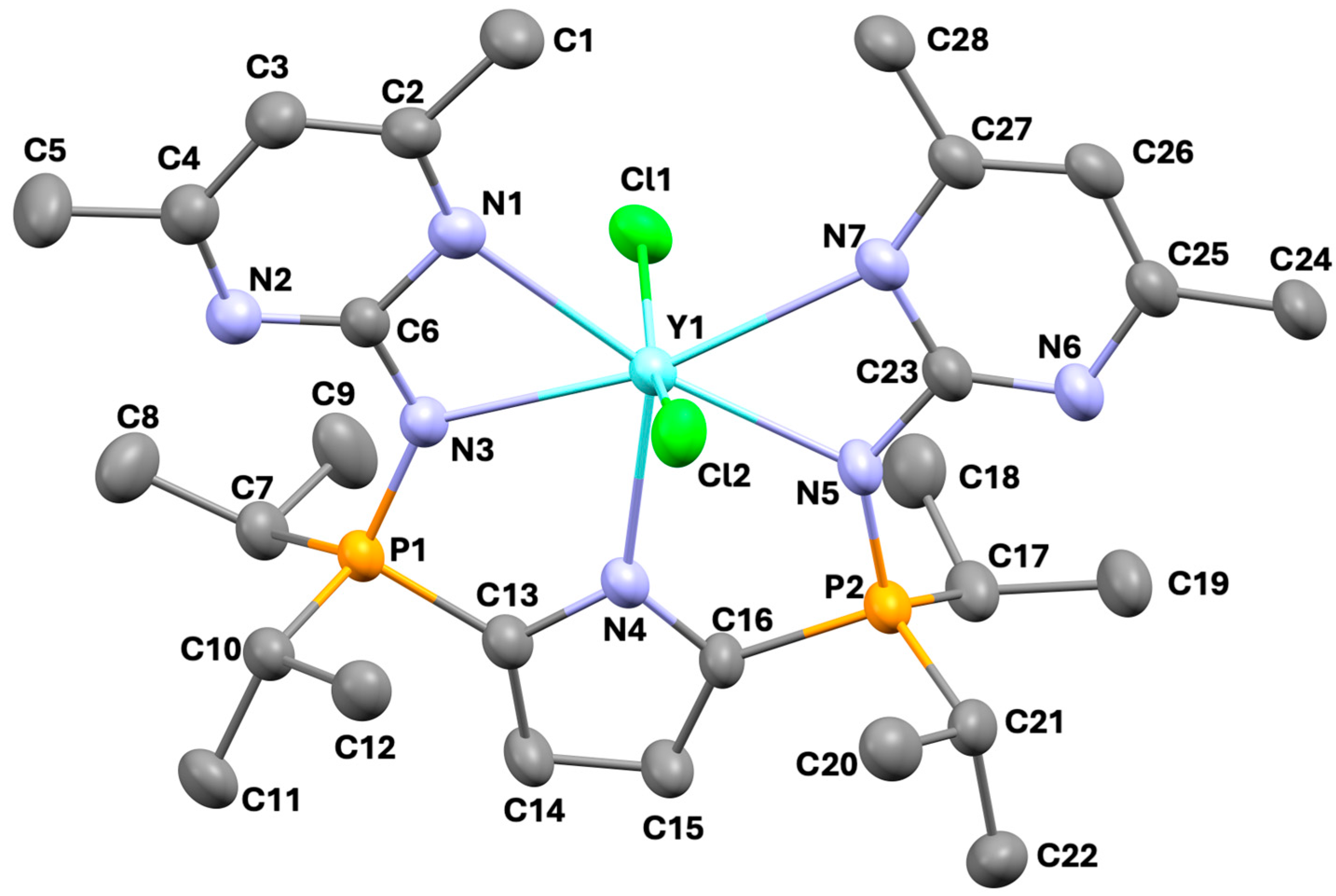
| Atom | Atom | Length/Å | Atom | Atom | Atom | Angle (°) |
|---|---|---|---|---|---|---|
| Y1 | Cl1 | 2.6072(8) | Cl2 | Y1 | Cl1 | 161.81(3) |
| Y1 | Cl2 | 2.6064(8) | N1 | Y1 | Cl1 | 82.66(6) |
| Y1 | N1 | 2.567(2) | N1 | Y1 | Cl2 | 86.77(6) |
| Y1 | N3 | 2.358(2) | N1 | Y1 | N7 | 114.17(8) |
| Y1 | N4 | 2.375(2) | N3 | Y1 | Cl1 | 91.83(7) |
| Y1 | N5 | 2.368(2) | N3 | Y1 | Cl2 | 93.83(7) |
| Y1 | N7 | 2.584(3) | N3 | Y1 | N1 | 53.83(8) |
| N3 | Y1 | N4 | 68.90(8) | |||
| LYCl2 Averages | N3 | Y1 | N5 | 138.46(8) | ||
| Y1 | Clave | 2.6028 ± 0.0004 | N3 | Y1 | N7 | 167.30(8) |
| Y1 | N1/N7 | 2.576 ± 0.009 | N4 | Y1 | Cl1 | 102.22(6) |
| Y1 | N3/N5 | 2.363 ± 0.005 | N4 | Y1 | Cl2 | 95.94(6) |
| N4 | Y1 | N1 | 122.70(8) | |||
| Literature Averages | N4 | Y1 | N7 | 123.07(8) | ||
| Y1 | Cl | 2.62 ± 0.02 | N5 | Y1 | Cl1 | 98.01(6) |
| Y1 | N3/N5 | 2.39 ± 0.02 | N5 | Y1 | Cl2 | 89.15(6) |
| Y1 | N4 | 2.345 ± 0.002 | N5 | Y1 | N1 | 167.40(9) |
| Y1 | N | 2.5126 ± 0.009 | N5 | Y1 | N4 | 69.58(8) |
| Lu1 | N1/N7 | 2.546 ± 0.002 | N5 | Y1 | N7 | 53.81(8) |
| Zr1 | N | 2.479 ± 0.008 | N7 | Y1 | Cl1 | 81.71(6) |
| Zr1 | Cl | 2.25 ± 0.05 | N7 | Y1 | Cl2 | 89.24(6) |
| Number | Atom 1 | Atom 2 | Length | Length-VdW | Symm. Op. 1 | Symm. Op. 2 |
|---|---|---|---|---|---|---|
| 1 | C3 | C15′ | 3.317 | −0.223 | x, y, z | −1/2 + x, 1−y, z |
| 2 | Cl2 | H5B′ | 2.598 | −0.422 | x, y, z | 1/2 − x, 1/2 − y, 1/2 − z |
| 3 | H22C | H5T′ | 2.161 | −0.239 | x, y, z | x, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trew, E.L.; Szucs, D.; Hayes, P.G. Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene. Molbank 2025, 2025, M2066. https://doi.org/10.3390/M2066
Trew EL, Szucs D, Hayes PG. Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene. Molbank. 2025; 2025(4):M2066. https://doi.org/10.3390/M2066
Chicago/Turabian StyleTrew, Emily L., David Szucs, and Paul G. Hayes. 2025. "Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene" Molbank 2025, no. 4: M2066. https://doi.org/10.3390/M2066
APA StyleTrew, E. L., Szucs, D., & Hayes, P. G. (2025). Dichloro[2,5-bis(diisopropylphosphorimidoyl-κN-(4,6-dimethylpyrimidine-κN))pyrrole-κN]yttrium(III)·toluene. Molbank, 2025(4), M2066. https://doi.org/10.3390/M2066






