N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(2,2,2-trichloroacetamido)benzamide
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayao, S.; Havera, H.J.; Strycker, W.G.; Leipzig, T.J.; Kulp, R.A.; Hartzler, H.E. New sedative and hypotensive 3-substituted 2, 4 (1H, 3H)-quinazolinediones. J. Med. Chem. 1965, 8, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Meuldermans, W.; Hendrickx, J.; Woestenborghs, R.; Van Peer, A.; Lauwers, W.; De Cree, J.; Heykants, J. Absorption, metabolism and excretion of ketanserin in man after oral administration. J. Arzneim. Forsch/Drug Res. 1988, 38, 789–794. [Google Scholar]
- Kakuta, H.; Tanatani, A.; Nagasawa, K.; Hashimoto, Y. Specific Nonpeptide Inhibitors of Puromycin-Sensitive Aminopeptidase with a 2,4(1H,3H)-Quinazolinedione Skeleton. Chem. Pharm. Bull. 2003, 51, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Petrov, J.S.; Andreev, G.N. Synthesis of 2,4(1H,3H)-Quinazolinedione and 3-Substituted 2,4(1H,3H)-Quinazolinediones. Org. Prep. Proced. Int. 2005, 37, 560–565. [Google Scholar] [CrossRef]
- Penchev, P. Computer-Assisted Interpretation of Molecular Spectra with the Aim of Elucidating the Structure of Organic Compounds. Ph.D. Thesis, University of Plovdiv “Paisii Hilendarski”, Plovdiv, Bulgaria, September 2016. [Google Scholar]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDBConstructing a Free Chemical Information System with Open-Source Components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, C.; Kuhn, S. NMRShiftDB–compound identification and structure elucidation support through a free community-built web database. Phytochem. 2004, 65, 2711–2717. [Google Scholar] [CrossRef] [PubMed]
- Penchev, P.N.; Schulz, K.-P.; Munk, M.E. INFERCNMR: A 13C NMR Interpretive Library Search System. J. Chem. Inf. Model. 2012, 52, 1513–1528. [Google Scholar] [CrossRef]
- Breitmaier, E. Recognition of Structural Fragments by NMR. In StructureElucidation by NMR in Organic Chemistry: A Practical Guide, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; p. 27. [Google Scholar]
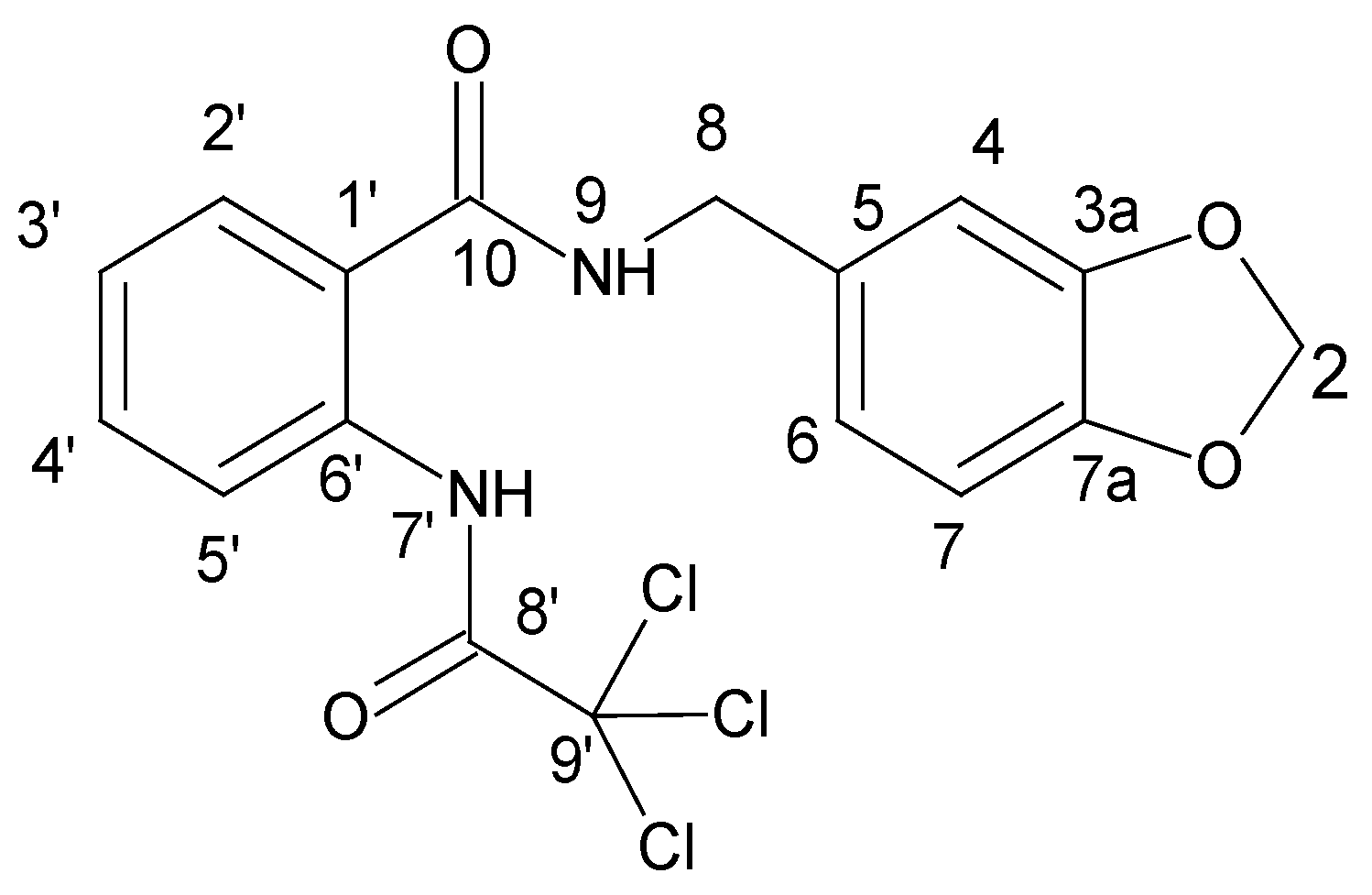
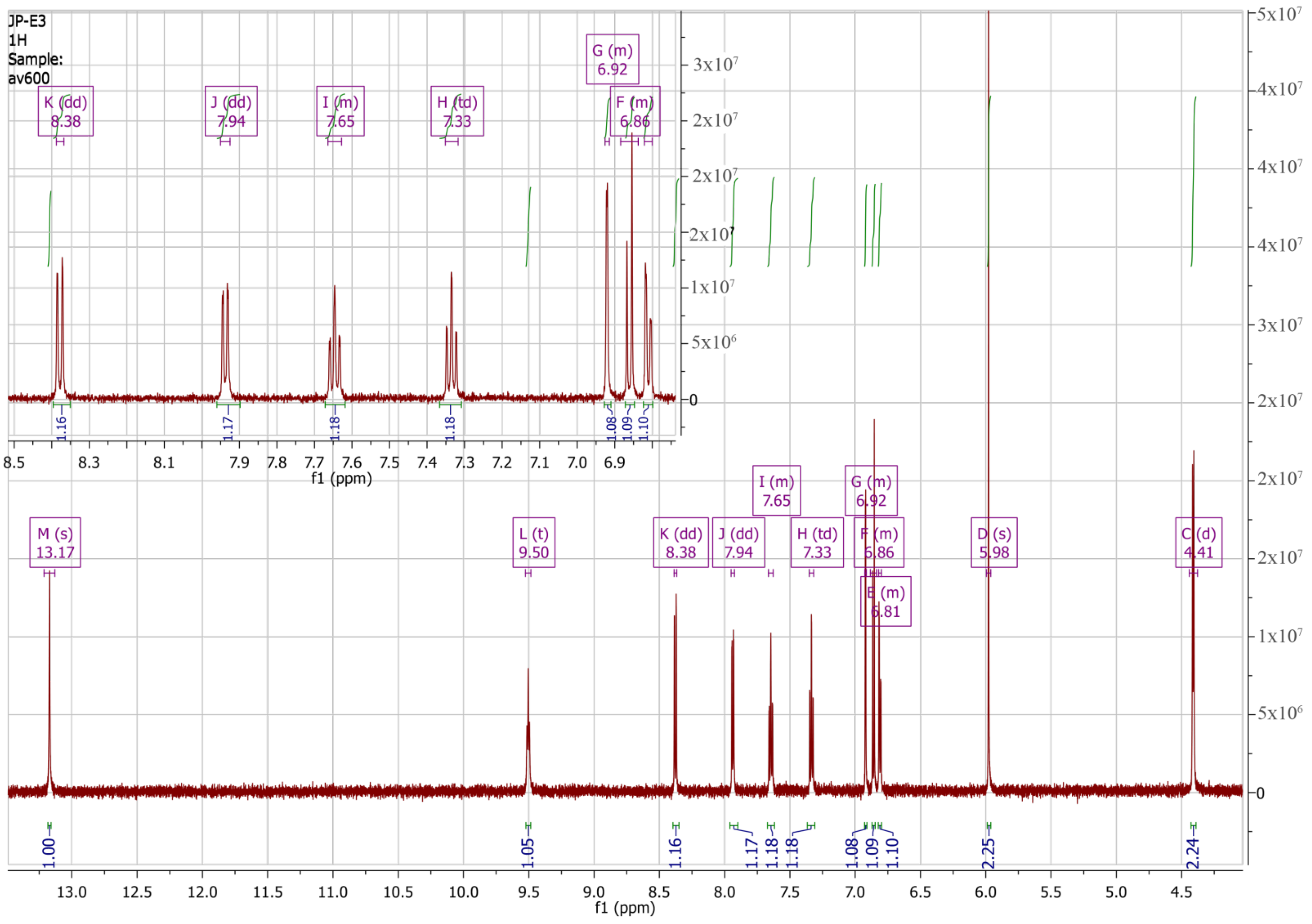
| Atom | δ (13C), ppm | DEPT b | δ (1H), ppm | Multiplicity (J, Hz) | 1H-1H COSY b | HMBC b |
|---|---|---|---|---|---|---|
| 1′ | 121.54 | C | ||||
| 2′ | 128.67 | CH | 7.94 | dd (7.9, 1.3) | 3′, 4′ d | 1′ d, 4′, 6′, 10 |
| 3′ | 125.24 | CH | 7.33 | td (7.7, 1.1) | 2′, 4′, 5′ d | 1′, 5′, 2′ c,4′ d, 6′ d |
| 4′ | 133.13 | CH | 7.65 | m | 2′ d, 3′, 5′ | 5′ c, 2′, 6′ |
| 5′ | 120.64 | CH | 8.38 | dd (8.2, 0.6) | 3′ d, 4′ | 1′, 3′, 6′ c, 10 d |
| 6′ | 137.55 | C | ||||
| 7′(NH) | 13.17 | s | ||||
| 8′(C=O) | 159.72 | C | ||||
| 9′ | 93.07 | C | ||||
| 2 | 101.22 | CH2 | 5.98 | s | 3a, 7a | |
| 3a | 147.60 | C | ||||
| 7a | 146.55 | C | ||||
| 4 | 108.37 | CH | 6.92 | m | 6 c, 7 d, 8 c | 6, 8, 7a |
| 5 | 132.92 | C | ||||
| 6 | 121.05 | CH | 6.81 | m | 4 c, 7, 8 d | 4, 7a, 8 |
| 7 | 108.45 | CH | 6.86 | m | 4 d, 6 | 3a, 5 |
| 8 | 42.82 | CH2 | 4.41 | d (6.0) | 4 c, 6 d, 9 | 4, 6, 5, 10 |
| 9(NH) | 9.50 | t (6.1) | 8 | 8 c, 10 | ||
| 10(C=O) | 168.41 | C |
| Carbon | Experimental δ (13C), ppm | Predicted δ (13C), ppm |
|---|---|---|
| C-1′ | 121.54 | 119.90 |
| C-2′ | 128.67 | 129.40 |
| C-3′ | 125.24 | 123.20 |
| C-4′ | 133.13 | 132.44 |
| C-5′ | 120.64 | 121.11 |
| C-6′ | 137.55 | 139.76 |
| C-8′ | 159.72 | 161.80 |
| C-9′ | 93.07 | 91.95 |
| C-10 | 168.41 | 168.50 |
| C-5 | 132.92 | 130.30 |
| C-4 | 108.37 | 108.00 |
| C-6 | 121.05 | 120.35 |
| C-7 | 108.45 | 108.50 |
| C-8 | 42.82 | 43.63 |
| C-3a | 147.60 | 147.70 |
| C-7a | 146.55 | 146.50 |
| C-2 | 101.22 | 101.18 |
| IR, cm−1 | Raman, cm−1 |
|---|---|
| 3318 (v(NH)) | 3076 (v(Csp2-H)) |
| 3244 (v(NH)) | 2942 (vas(CH2)) |
| 3075 (v(Csp2-H)) | 1718 (v(C=O)) |
| 2923 (vas(CH2)) | 1707 (v(C=O)) |
| 1718 (v(C=O)) | 1599 (v(C=C)) |
| 1707 (v(C=O)) | 1587 (v(C=C)) |
| 1601 (v(C=C)) | 1516 (v(C=C)) |
| 1587 (v(C=C)) | 1502 (v(C=C)) |
| 1518 (v(C=C)) | 1448 (v(C=C)) |
| 1503 (v(C=C)) | |
| 1490 (v(C=C)) | |
| 1447 (v(C=C)) | |
| 764 (ϒ(C-H) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penchev, P.; Stoitsov, D. N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(2,2,2-trichloroacetamido)benzamide. Molbank 2025, 2025, M2052. https://doi.org/10.3390/M2052
Penchev P, Stoitsov D. N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(2,2,2-trichloroacetamido)benzamide. Molbank. 2025; 2025(3):M2052. https://doi.org/10.3390/M2052
Chicago/Turabian StylePenchev, Plamen, and Dimitar Stoitsov. 2025. "N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(2,2,2-trichloroacetamido)benzamide" Molbank 2025, no. 3: M2052. https://doi.org/10.3390/M2052
APA StylePenchev, P., & Stoitsov, D. (2025). N-[(2H-1,3-benzodioxol-5-yl)methyl]-2-(2,2,2-trichloroacetamido)benzamide. Molbank, 2025(3), M2052. https://doi.org/10.3390/M2052






