Three Hypoxanthine Derivatives from the Marine Cyanobacterium Okeania hirsuta
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Collection of Cyanobacteria
3.3. Isolation of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus Okeania gen. nov. (Oscillatoriales, Cyanoprokaryota). J. Phycol. 2013, 49, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Zhang, B.T.; Shinjo, A.; Kamiya, M.; Nagai, H.; Uchida, H.; Araki, Y.; Nishikawa, T.; Satake, M. 7-Epi-30-methyloscillatoxin D from an Okinawan cyanobacterium Okeania hirsuta. Nat. Prod. Commun. 2023, 18, 1–5. [Google Scholar]
- Tidgewell, K.; Clark, B.R.; Gerwick, W.H. 2.06-The natural products chemistry of cyanobacteria. In Comprehensive Natural Products, 2nd ed.; Liu, H.W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 141–188. [Google Scholar]
- Umeda, K.; Kurisawa, N.; Jeelani, G.; Nozaki, T.; Suenaga, K.; Iwasaki, A. Isolation and structure determination of a new analog of polycavernosides from marine Okeania sp. cyanobacterium. Beilstein J. Org. Chem. 2024, 20, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, A.; Taguchi, R.; Jeelani, G.; Nozaki, T.; Suenaga, K.; Iwasaki, A. Kagimminols A and B, cembrene-type diterpenes from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2024, 87, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Jeelani, G.; Nozaki, T.; Iwasaki, A. Amantamide C, an antitrypanosomal linear lipopeptide from a marine Okeania sp. cyanobacterium. ACS Omega 2024, 9, 36795–36801. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.A.J.; Hough, A.; Wright, T.; Lax, N.; Faruk, A.; Fofie, C.K.; Simcik, R.D.; Cavanaugh, J.E.; Kolber, B.J.; Tidgewell, K.J. Isolation and bioassay of linear veraguamides from a marine cyanobacterium (Okeania sp.). Molecules 2025, 30, 680. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, M.; Kurisawa, N.; Umeda, K.; Jeelani, G.; Agusta, A.L.; Nozaki, T.; Suenaga, K. Ouhanamide, an antiparasitic linear lipopeptide from a marine Okeania sp. cyanobacterium. J. Nat. Prod. 2025, 88, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Kurisawa, N.; Umeda, K.; Jeelani, G.; Agusta, A.L.; Nozaki, T.; Suenaga, K. Sealgamide: An antitrypanosomal lipopeptide isolated from a marine Okeania sp. cyanobacterium. J. Nat. Prod. 2025, 88, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Tojo, S.; Natsume, N.; Niino, T.; Iwasaki, A.; Suenaga, K.; Ohno, O.; Teruya, T. Hedopeptolide, a NO production inhibitor from the marine cyanobacterium Okeania sp. J. Nat. Prod. 2025, 88, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.T.; Nishino, H.; Kawabe, R.; Kamio, M.; Watanabe, R.; Uchida, H.; Satake, M.; Nagai, H. N-Desmethylmajusculamide B, a lipopeptide isolated from the Okinawan cyanobacterium Okeania hirsuta. Biosci. Biotech. Biochem. 2024, 88, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kawabe, R.; Kamio, M.; Uchida, H.; Matsuura, N.; Mori-Yasumoto, K.; Satake, M.; Nagai, H. Okeaniazole A: Thiazole-containing cyclopeptide from the marine cyanobacterium Okeania hirsuta with antileishmanial activity. Tetrahedron Lett. 2024, 150, 155284. [Google Scholar] [CrossRef]
- Nishino, H.; Zhang, B.T.; Uchida, H.; Kamio, M.; Nagai, H.; Satake, M. (10 E, 15 Z)-12-(Dimethylsulfonio)-9, 13-dihydroxyoctadeca-10, 15-dienoate. Molbank 2024, 2024, M1784. [Google Scholar] [CrossRef]
- Bartl, T.; Zacharová, Z.; Sečkářová, P.; Kolehmainen, E.; Marek, R. NMR quantification of tautomeric populations in biogenic purine bases. Eur. J. Org. Chem. 2009, 2009, 1377–1383. [Google Scholar] [CrossRef]
- Qi, S.H.; Zhang, S.; Huang, H. Purine alkaloids from the South China Sea gorgonian Subergorgia suberosa. J. Nat. Prod. 2008, 71, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Schmalle, H.W.; Hänggi, G.; Dubler, E. Structure of hypoxanthine. Acta. Cryst. C. 1988, 44, 732–736. [Google Scholar] [CrossRef]
- Srinivasan, S.; Torres, A.G.; Ribas de Pouplana, L. Inosine in biology and disease. Genes 2021, 12, 600. [Google Scholar] [CrossRef] [PubMed]
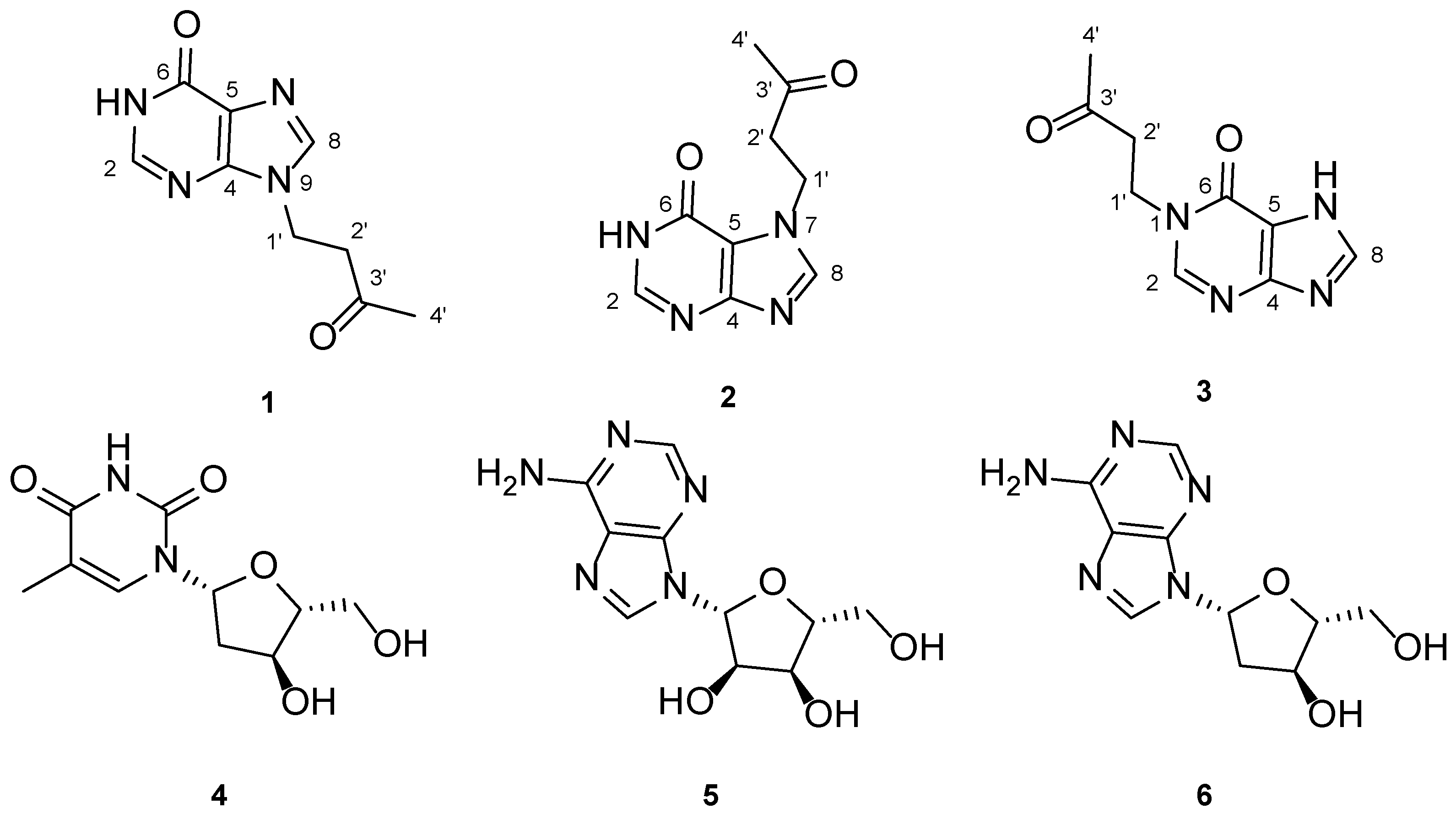
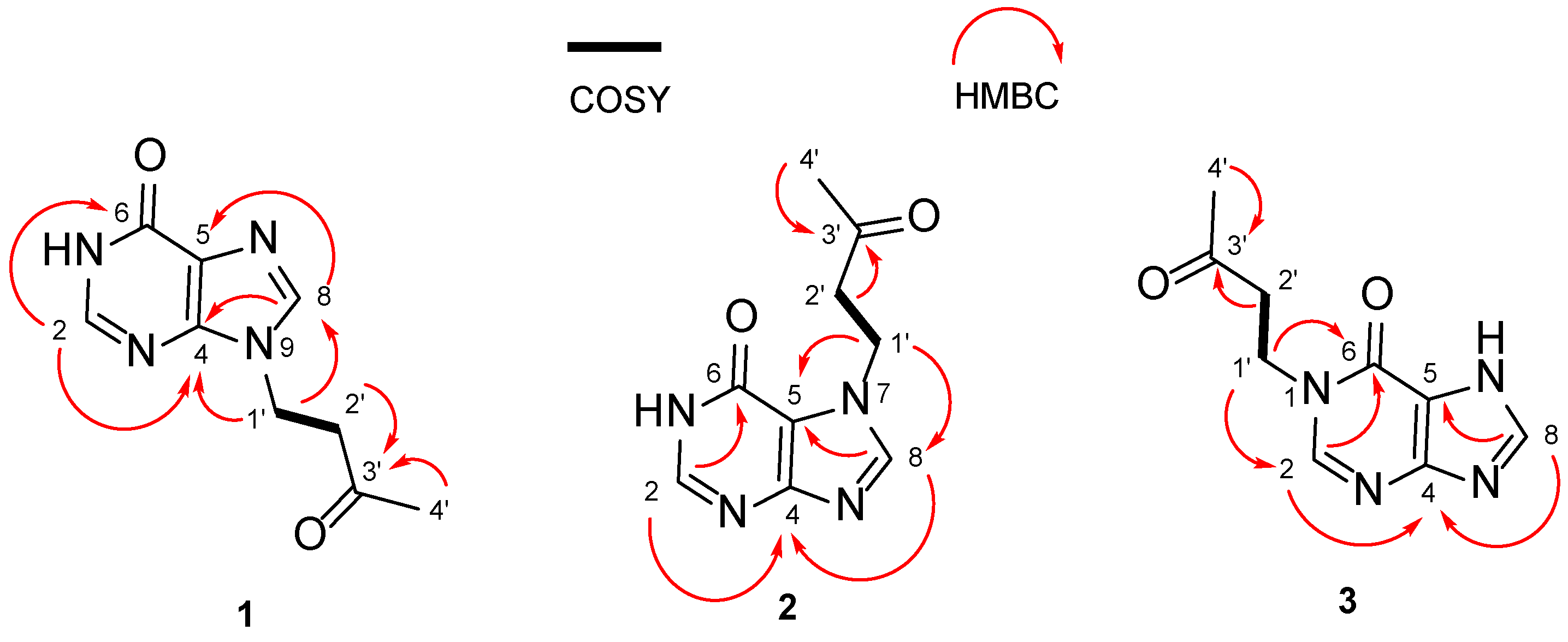
| 1 (in DMSO-d6) | 2 (in DMSO-d6) | 3 (in Acetic Acid-d4) | ||||
|---|---|---|---|---|---|---|
| No. | δH a (J in Hz) | δC b, type | δH a (J in Hz) | δC b, type | δH c (J in Hz) | δC d, type |
| 1′ | 4.28, dd (6.7, 6.7) | 38.3, CH2 | 4.45, dd (6.7, 6.7) | 41.4, CH2 | 4.37, dd (6.4, 6.4) | f 43.6, CH2 |
| 2′ | 3.08, dd (6.7, 6.7) | 42.3, CH2 | 3.11, dd (6.7, 6.7) | 43.5, CH2 | 3.12, dd (6.4, 6.4) | f 42.0, CH2 |
| 3′ | 206.2, C | e 206.2, C | e 209.1, C | |||
| 4′ | 2.10, s | 29.8, CH3 | 2.09, s | 29.8, CH3 | 2.16, s | f 29.7, CH3 |
| 2 | 8.01, s | 145.8, CH | 7.95, s | 144.4, CH | 8.57, s | f 150.1, CH |
| 4 | 148.3, C | e 157.3, C | e 153.7, C | |||
| 5 | 123.9, C | e 114.8, C | e 117.6, C | |||
| 6 | 157.1, C | e 154.3, C | e 156.8, C | |||
| 8 | 7.98, s | 140.2, CH | 8.15, s | 144.1, CH | 8.41, s | f 143.5, CH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabe, R.; Zhang, B.; Watanabe, R.; Uchida, H.; Satake, M.; Nagai, H. Three Hypoxanthine Derivatives from the Marine Cyanobacterium Okeania hirsuta. Molbank 2025, 2025, M2051. https://doi.org/10.3390/M2051
Kawabe R, Zhang B, Watanabe R, Uchida H, Satake M, Nagai H. Three Hypoxanthine Derivatives from the Marine Cyanobacterium Okeania hirsuta. Molbank. 2025; 2025(3):M2051. https://doi.org/10.3390/M2051
Chicago/Turabian StyleKawabe, Ryoya, Botao Zhang, Ryuichi Watanabe, Hajime Uchida, Masayuki Satake, and Hiroshi Nagai. 2025. "Three Hypoxanthine Derivatives from the Marine Cyanobacterium Okeania hirsuta" Molbank 2025, no. 3: M2051. https://doi.org/10.3390/M2051
APA StyleKawabe, R., Zhang, B., Watanabe, R., Uchida, H., Satake, M., & Nagai, H. (2025). Three Hypoxanthine Derivatives from the Marine Cyanobacterium Okeania hirsuta. Molbank, 2025(3), M2051. https://doi.org/10.3390/M2051






