5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one
Abstract
1. Introduction
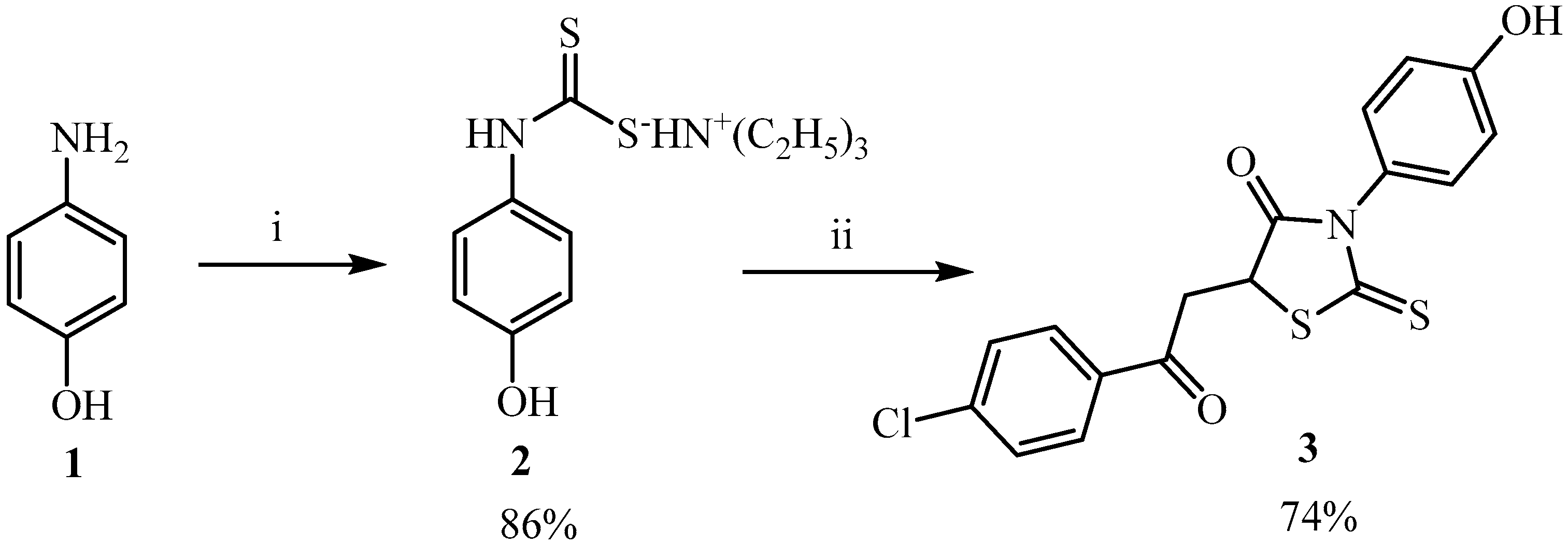
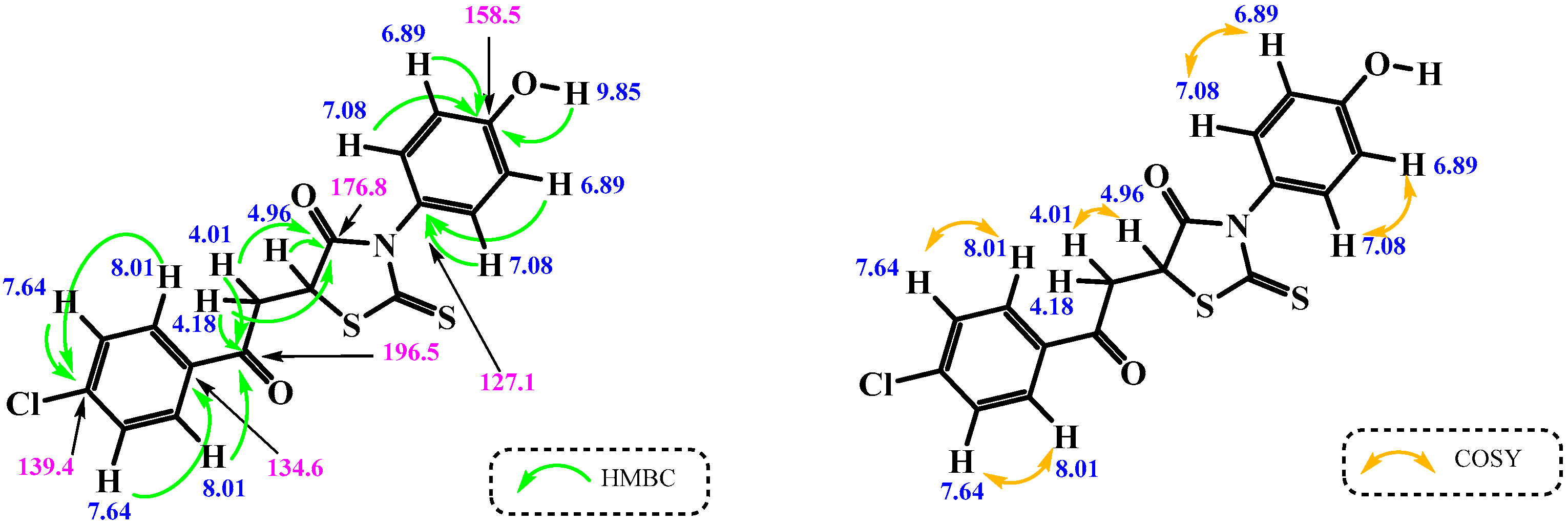
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Triethylammonium (4-hydroxyphenyl)dithiocarbamate (2)
3.3. Synthesis of 5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one (3)
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaminskyy, D.; Kryshchyshyn, A.; Lesyk, R. Recent developments with rhodanine as a scaffold for drug discovery. Expert Opin. Drug Discov. 2017, 12, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Chaurasyia, A.; Chawla, P.; Monga, V.; Singh, G. Rhodanine derivatives: An insight into the synthetic and medicinal perspectives as antimicrobial and antiviral agents. Chem. Biol. Drug Des. 2023, 101, 500–549. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, J.; Tuszewska, H.; Trotsko, N. Anticancer profile of rhodanines: Structure–Activity Relationship (SAR) and molecular targets—A review. Molecules 2022, 27, 3750. [Google Scholar] [CrossRef] [PubMed]
- Abusetta, A.; Alumairi, J.; Alkaabi, M.Y.; Al Ajeil, R.; Shkaidim, A.A.; Akram, D.; Pajak, J.; Ghattas, M.; Atatreh, N.; Al Neyadi, S.S. Design, synthesis, in vitro antibacterial activity, and docking studies of new rhodanine derivatives. Open J. Med. Chem. 2020, 10, 15–34. [Google Scholar] [CrossRef]
- Horishny, V.; Kartsev, V.; Geronikaki, A.; Matiychuk, V.; Petrou, A.; Glamoclija, J.; Petrou, A.; Sokovic, M. 5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-ylalkancarboxylic acids as antimicrobial agents: Synthesis, biological evaluation, and molecular docking studies. Molecules 2020, 25, 1964. [Google Scholar] [CrossRef] [PubMed]
- Gentili, V.; Turrin, G.; Marchetti, P.; Rizzo, S.; Schiuma, G.; Beltrami, S.; Cristofori, V.; Illuminati, D.; Comapnin, V.; Trapella, C.; et al. Synthesis and biological evaluation of novel rhodanine-based structures with antiviral activity toward HHV-6 virus. Bioorg. Chem. 2022, 119, 105518. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; He, X.; Xu, W.; Wang, C.; Lan, Q.; Hua, C.; Wang, K.; Lu, L.; Jiang, S. The analogs of furanyl methylidene rhodanine exhibit broad-spectrum inhibitory and inactivating activities against enveloped viruses, including SARS-CoV-2 and its variants. Viruses 2022, 14, 489. [Google Scholar] [CrossRef] [PubMed]
- Szczepański, J.; Khylyuk, D.; Korga-Plewko, A.; Michalczuk, M.; Mańdziuk, S.; Iwan, M.; Trotsko, N. Rhodanine–piperazine hybrids as potential VEGFR, EGFR, and HER2 targeting anti-breast cancer agents. Int. J. Mol. Sci. 2024, 25, 12401. [Google Scholar] [CrossRef] [PubMed]
- Buzun, K.; Kryshchyshyn-Dylevych, A.; Senkiv, J.; Roman, O.; Gzella, A.; Bielawski, K.; Bielawska, A.; Lesyk, R. Synthesis and anticancer activity evaluation of 5-[2-chloro-3- (4-nitrophenyl)-2-propenylidene]-4-thiazolidinones. Molecules 2021, 26, 3057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ye, C.; Chen, S.; Fang, Y.; Zhang, Y.; Zhang, T. Rhodanine derivatives containing 5-aryloxypyrazole moiety as anti-inflammatory and anticancer agents. Chem. Biodiversity 2024, 21, e202301844. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.-S.; Lee, Y.; Yang, J.; Kim, S.K.; Shin, Y.; Kim, H.-J.; Choi, J.H.; Im, Y.J.; Kim, M.-J.; Lee Yu, K.; et al. Characterization of rhodanine derivatives as potential disease-modifying drugs for experimental mouse osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, L.; Feng, H.; Liu, B. Molecular engineering of rhodamine dyes for highly efficient D-π-A organic sensitizer. Dyes Pigm. 2018, 156, 53–60. [Google Scholar] [CrossRef]
- Syiemlieh, C.C.; Ilango, B.; Kothandaraman, R.; Venkatakrishnan, P.; Velusamy, M.; Kathiravan, A. Delving into the role of a conjugated rhodanine acceptor in D–D′–A dyes for photovoltaic applications. J. Phys. Chem. C 2025, 129, 5821–5832. [Google Scholar] [CrossRef]
- Omar, M.T.; Kandeel, K.A.; Youssef, A.S.A. The (E)/(Z)-ratio in the reaction of 5-(2-aryl-2-oxoethyl)-2-thioxo-4-oxo-1,3-thiazolidines with bromine. Monatsh. Chem. 1995, 126, 439–446. [Google Scholar] [CrossRef]
- Takahashi, T. Spin-spin coupling constants for methylene groups adjacent to carbonyl groups. Tetrahedron Lett. 1964, 5, 565–572. [Google Scholar] [CrossRef]
- Brand, W.W.; Lovell, J.B. Insect control compounds. U.S. Patent 4,004,019, 18 January 1977. [Google Scholar]
- Hall, B.; Praveen, K.; Decorte, B. Nifuroxazide analogs and therapeutic uses thereof. WO 2019/126568 A1, 27 June 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotsko, N. 5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one. Molbank 2025, 2025, M2049. https://doi.org/10.3390/M2049
Trotsko N. 5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one. Molbank. 2025; 2025(3):M2049. https://doi.org/10.3390/M2049
Chicago/Turabian StyleTrotsko, Nazar. 2025. "5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one" Molbank 2025, no. 3: M2049. https://doi.org/10.3390/M2049
APA StyleTrotsko, N. (2025). 5-[2-(4-Chlorophenyl)-2-oxoethyl]-3-(4-hydroxyphenyl)-2-thioxo-1,3-thiazolidin-4-one. Molbank, 2025(3), M2049. https://doi.org/10.3390/M2049





