Abstract
In this report, we describe the synthesis of a compound derived from the natural compound celastrol, which is connected to a phthalimide moiety via an ester linkage. The compound was fully characterized by proton (1H), carbon-13 (13C), heteronuclear single-quantum coherence (HSQC), and distortionless enhancement by polarization transfer (DEPT) NMR. Ultraviolet–visible spectroscopy (UV-Vis), Fourier-transform infrared (FTIR), and elementary analysis were also performed.
1. Introduction
In recent years, celastrol (1) has emerged as a compound of considerable interest in the search for new therapeutic agents, owing to its broad pharmacological potential. Isolated from the roots of Tripterygium wilfordii, a key species in traditional Chinese medicine (TCM) with a long-standing history, celastrol has transitioned from a folk remedy to a subject of rigorous biomedical research. Although its medicinal use dates back centuries, the scientific community has only recently begun to elucidate the full extent of its biological activity, primarily through mechanistic studies conducted over the past decade.
Celastrol exhibits a broad spectrum of pharmacological effects, including anti-inflammatory [1], antioxidant [2], cardioprotective [3], and anti-rheumatic properties [4]. Moreover, it has demonstrated anticancer activity [5], neuroprotective potential in neurodegenerative conditions [6], and anti-obesity effects [7]. A particularly compelling aspect of celastrol’s bioactivity lies in its modulation of critical cellular signaling pathways involved in disease development. It has been shown to suppress the activation of NF-κB [8], disrupt the Hsp90–Cdc37 complex [9], and inhibit the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) [10], all of which are linked to inflammatory and proliferative diseases.
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by persistent inflammation and progressive joint deterioration. With a global prevalence estimated between 0.25% and 1% [11], RA remains a significant public health burden. Despite considerable efforts to manage its symptoms, a definitive cure has yet to be found. The pathophysiology of RA is multifactorial, involving a complex interplay among immune cells (e.g., T cells, macrophages, fibroblasts) and molecular mediators [12]. Among these, calcium ions (Ca2+) play a central role in driving autoimmune responses and maintaining chronic inflammation [13]. SERCA, a key regulator of calcium homeostasis, has been studied for its inhibitory abilities not only in cancer research, where it leads to increased intracellular calcium, ATP depletion, and cell death [14], but also as a therapeutic target in inflammatory diseases such as RA [10,15]. Celastrol’s ability to target SERCA has attracted considerable attention due to its observed effects in reducing bone erosion and suppressing inflammatory arthritis in preclinical studies [10].
Although celastrol holds considerable therapeutic promise, its clinical translation is hindered by several challenges, such as its notably high toxicity, poor aqueous solubility, and chemical instability. These limitations have prompted extensive efforts in medicinal chemistry to develop structurally modified derivatives with improved drug-like properties (Figure 1, panel ii). Strategies such as esterification and amidation of the C29-carboxylic acid group (Figure 1, panel i) have been applied to enhance water solubility, reduce toxicity, and explore novel pharmacological activities [16,17,18].
Phthalimide-based scaffolds have garnered increasing interest in recent years among medicinal chemists due to their remarkable versatility and broad-spectrum biological activities [19]. These nitrogen-containing heterocyclic compounds, characterized by a phthalimide core, have served as privileged scaffolds in the development of new pharmacophores with diverse therapeutic applications. The structural rigidity and electron-withdrawing characteristics of the phthalimide ring system contribute to its favorable pharmacokinetic and pharmacodynamic properties, making it a suitable candidate for drug design [20]. Numerous studies have reported the antitumor activity of phthalimide derivatives, particularly through mechanisms involving the induction of apoptosis, inhibition of angiogenesis, and disruption of cell cycle progression [21]. For instance, thalidomide and its analogues, lenalidomide and pomalidomide, are clinically approved immunomodulatory agents that have revolutionized treatment paradigms for multiple myeloma and other hematologic malignancies [22].
Beyond oncology, phthalimide compounds have demonstrated promising antitubercular and antimalarial effects, often attributed to their ability to disrupt vital enzymatic pathways in Mycobacterium tuberculosis [23] and Plasmodium species [24], respectively. Their structural framework has also been utilized in the synthesis of potent antioxidants, which scavenge free radicals and mitigate the oxidative stress implicated in various chronic diseases. Furthermore, antimicrobial activity against both Gram-positive and Gram-negative bacterial strains has been documented, with several derivatives showing potential as alternatives to conventional antibiotics [25].
Additionally, the anti-inflammatory properties of phthalimide derivatives have been extensively investigated [26], with many compounds being observed to be capable of modulating key mediators such as prostaglandins, cytokines, and nitric oxide. These agents are being explored for the treatment of inflammatory and autoimmune disorders, including RA and inflammatory bowel disease. Taken together, the multifaceted biological profile of phthalimide-based moieties, combined with their synthetic accessibility and amenability to structural modification, positions them as a highly valuable chemotype in the ongoing search for novel therapeutic agents. Recently, a derivative of celastrol containing phthalimide moiety was synthesized via esterification and analyzed for its in vitro anticancer activity [27].
This study describes the synthesis of a new celastrol derivative whose scaffold has been linked to phthalimide derivatives (Scheme 1), which was designed and fully characterized by NMR, IR, UV-Vis, and elementary analyses.
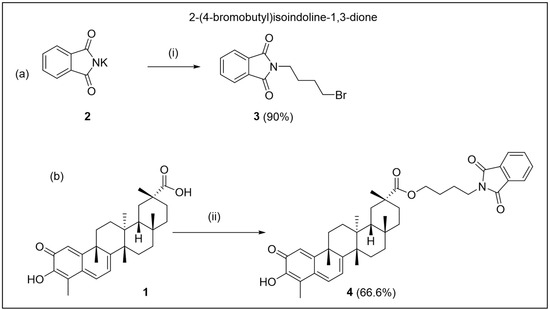
Scheme 1.
(a) Synthesis of 2-(4-bromobutyl)isoindoline-1,3-dione 3 (i) DMF, 1, 4-dibromobutane, 24 h, room temperature; (b) synthesis of 4-(1,3-dioxoisoindolin-2-yl)butyl (2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylate (ii); 3 (6 equiv.), K2CO3 (6 equiv.), DMF, room temperature; 12 h.
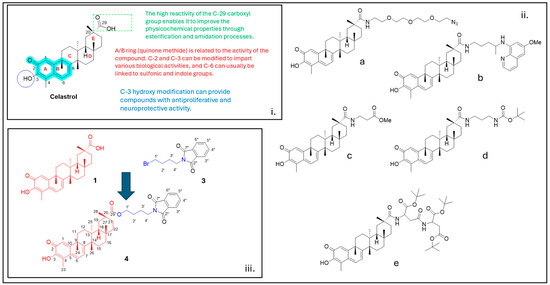
Figure 1.
i. Functional groups of celastrol (1) that are important for its biological activity; ii. Example of celastrol derivatives (a) [28], (b) [17], and (c–e) [16] reported in the literature; iii. Chemical structures of 2-(4-bromobutyl)isoindoline-1,3-dione (3) and compound 4 are reported in the inset of the figure.
2. Results and Discussion
The synthetic pathway toward 4-(1,3-dioxoisoindolin-2-yl)butyl(2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylate was initiated by the preparation of the precursor (3), as outlined in Scheme 1a.
Isoindoline-1,3-dione, also known as phthalimide, is widely recognized in synthetic organic chemistry due to its multifunctional utility in fields of pharmaceuticals, agrochemicals, and materials science. This compound acts as an amine synthon (-NH2 equivalent), enabling efficient access to primary amines. Following a reported procedure [29], 2-(4-bromobutyl)-isoidoline-1,3-dione 3 was prepared by the reaction of isoindoline-1,3-dione potassium salt 2 with 1,4-dibromobutane in DMF. The identity of 3 was confirmed by 1H-, 13C-NMR, and DEPT-135 spectra (Supplementary Materials, Figures S1–S3).
Compound 3 was subsequently used in a coupling reaction to obtain the final ester product. The transformation was carried out under carbonate-mediated activation conditions, employing a modified protocol described by Jiang et al. [30]. Specifically, celastrol was dissolved in DMF and treated with potassium carbonate and a six-equivalents compound 3 (Scheme 1, step b), resulting in the formation of compound 4 with high yield (66.6%).
The structure of compound 4 was elucidated by NMR, IR, UV-Vis and elementary analyses. Concerning NMR analysis, aromatics proton signals corresponding to position 3′′–6′′ appeared in the δH 7.7–7.8 ppm region (Figures S4 and S5), while the methylene group gave rise to peaks between δH 3.5 and 4 ppm in the 1H-NMR spectrum, consistent with the proposed molecular framework. Regarding the 13C-NMR spectrum, the disappearance of the characteristic carboxylic acid carbon peak at 182 ppm (present in compound 1), replaced by a new signal at 178.4 ppm attributable to the amide carbon, confirmed the successful transformation (Figure S6).
Further structural assignments were supported by 2D NMR experiments, including Heteronuclear single-quantum coherence spectroscopy (HSQC) and distortionless enhancement by polarization transfer (DEPT-135, DEPT-90 and DEPT-45), which enabled the classification of carbon environments in compound 4 (Table S1; Supplementary Figures S7–S10). A total of 41 carbon signals were observed, corresponding to six methyl groups, eleven methylene carbons, eight methines, and sixteen quaternary carbons.
The IR spectrum of compound 4 showed prominent bands at 3427 cm−1 (O-H stretch), 2954 and 2926 cm−1 (C-H stretch), and 1714 cm−1 (amide C=O stretch of phthalimide moiety), along with a peak at 1595 cm−1 corresponding to N-H bending (Figures S11 and S12). The UV-Vis spectral analysis further confirmed the compound’s identity, displaying a strong absorption band in the range of 228–242 nm and a weaker band at 426 nm, corresponding to an n→π* transition) (Figure S13).
3. Materials and Methods
3.1. Chemistry
Silica gel (FCP 230–400 mesh) was used for column chromatography. Thin-layer chromatography (TLC) was carried out on Merck precoated silica gel 60 F254 plates (Merck, Darmstadt, Germany) and visualized with phosphomolybdic acid, iodine, or a UV lamp. All reagents were purchased from Bide Pharmatech., Ltd. (Shanghai, China).
1H-NMR and 13C-NMR spectra were recorded in CDCl3 at 25 °C on a Bruker Ascend®-600 (Magnet System 600′54 Ascend LH, San Jose, CA, USA) NMR spectrometer (600 MHz for 1H and 150 MHz for 13C). All chemical shifts (δ) are reported in the standard δ notation of parts per million (ppm) using the peak of the residual proton signals of CDCl3 as an internal reference (CDCl3, δH 7.26 ppm, δC 77.2 ppm).
UV-Vis analysis was performed by a Shimadzu UV–2600 (Osaka, Japan) with a 1 cm quartz cell and a slit width of 2.0 nm. The analysis was carried out using wavelengths in the range of 200–700 nm.
IR spectra were obtained using a Shimadzu, IRAffinity-1S Fourier transform infrared spectrometer (Osaka, Japan).
3.2. 2-(4-Bromobutyl) isoindoline-1, 3-dione (3)
A mixture of phthalimide potassium (2.0 g, 10.7 mmol) and 1, 4-dibromobutane (2.8 g, 12.9 mmol) in dry DMF (25 mL) was stirred at room temperature for 26 h. After that, the reaction mixture was extracted with CH2Cl2 and washed with water. The combined organic layer was dried over anhydrous MgSO4, filtered, and evaporated in vacuo. The resulting residue was recrystallized in distilled water to afford 3 as a white solid with 90% yield. m.p.: 75–77 °C. 1H-NMR (600 MHz, CD3Cl): δ ppm: 7.5–7.83 (m, 2H), 7.71–7.72 (m, 2H), 3.72 (t, J = 6.7 Hz, 2H), 3.44 (t, J = 6.6 Hz, 2H), 1.90 (m, 2H), 1.83 (m, 2H); 13C-NMR (150 MHz, CDCl3) δ ppm: 168.51, 134.13, 132.18, 123.40, 37.09, 32.92, 29.97, 27.37. ESI-MS: ([M + H]+): 283.4.
The spectral characteristics are consistent with those of 3 in the literature [29].
3.3. 4-(1,3-Dioxoisoindolin-2-yl)butyl (2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylate (4)
Potassium carbonate (six equivalents) was added to a solution of celastrol (45 mg, 0.10 mmol) in dichloromethane (6 mL), and the mixture was stirred at room temperature for 12 h. The mixture was washed three times with water, and the organic phase was collected, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to yield the crude product. Purification was achieved via chromatography on a silica gel column (eluents: hexane/ethyl acetate 2:1). Yield 66.6%, 1H NMR (600 MHz, CDCl3) δ ppm:7.84 (dd, J = 5.4, 3.0 Hz, 2H), 7.71 (dd, J = 5.4, 3.0 Hz, 2H), 6.99 (d, J = 1.4 Hz, 1H), 6.50 (d, J = 1.6 Hz, 1H), 6.33 (d, J = 7.2 Hz, 1H), 4.04–3.82 (m, 2H), 3.70 (t, J = 7.1 Hz, 2H), 2.38 (d, J = 15.9 Hz, 1H), 2.19 (s, 3H), 2.12–1.83 (m, 4H), 1.80–1.72 (m, 5H), 1.69–1.46 (m, 7H), 1.42 (s, 3H), 1.38–1.32 (m, 1H), 1.24 (s, 3H), 1.15 (s, 3H), 1.07 (s, 3H), 0.97–0.92 (m, 1H), 0.52 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ ppm: 10.36 (CH3), 18.59 (CH3), 21.75 (CH3), 25.48, 26.02, 27.35, 28.74, 29.76, 29.80, 29.89, 29.94, 30.64, 31.03, 31.70, 32.89, 33.58, 34.90, 36.47, 37.68, 38.32, 39.55, 40.51, 43.04, 44.36, 45.16, 63.85, 117.20, 118.25, 119.67, 123.41, 127.51, 132.17, 134.09, 134.19, 146.12, 164.80, 168.45, 170.07, 178.33, 178.46; UV-Vis (CH2Cl2) peaks 228 and 424 nm; IR (FTIR) 3332, 2931, 2870, 2360, 2106, 1643, 1589, 1442, 1288 and 1103 cm−1. Calc. for C41H49NO6: C, 75.2; H, 7.4; N, 2.1; O, 14.3
4. Conclusions
In this study, a celastrol derivative was synthesized and evaluated for its potential applications in cancer therapy and anti-inflammatory treatment. The compound was thoroughly characterized using NMR, UV-Vis, IR, and elementary analysis.
Supplementary Materials
The following supporting information is available online. Figure S1: 1H-NMR spectrum (CDCl3, 600 MHz) of compound 3; Figure S2: 13C-NMR spectrum (CDCl3, 150 MHz) of compound 3; Figure S3: DEPT-135 spectrum (CDCl3, 150 MHz) of compound 3; Figure S4: 1H-NMR spectrum (CDCl3, 600 MHz) of compound 4; Figure S5: An expanded view of 1H-NMR spectrum (CDCl3, 600 MHz) of compound 4, Figure S6: 13C-NMR spectrum (CDCl3, 150 MHz) of compound 4; Figure S7: DEPT-135 spectrum (CDCl3, 150 MHz) of compound 4; Figure S8: DEPT-90 spectrum (CDCl3, 150 MHz) of compound 4; Figure S9: HSQC spectrum of compound 4; Figure S10: An expanded view of HSQC spectrum compound 4; Table S1: 1H and 13C-nuclear magnetic spectroscopy (NMR) chemical shifts and the structure of compound 4; Figure S11: IR spectrum (KBr) of compound 1; Figure S12: IR spectrum (FTIR) of compound 4; Figure S13: UV-Vis spectrum of compound 4 (range 200–600 nm in CH2Cl2).
Author Contributions
Investigation, Z.C.; data curation, Z.C. and K.F.L.; writing—original draft preparation, Z.C. and K.F.L.; writing—review and editing, Z.C., K.F.L., C.C., and P.C.; supervision, C.C. and P.C.; funding acquisition, C.C. and P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FDCT grants from Macao Science and Technology University to PC (Project Code: 0005-2023-RIA1) and Ministry of Science and Technology in Taiwan (Grant numbers MOST 110-2113-M-039-001 and MOST 111-2221-E-039-009) to CC.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. Adding the relevant information “Data Availability Statement: Data is contained within the article or supplementary material.” This change does not affect the scientific content of the article.
References
- Kannaiyan, R.; Shanmugam, M.K.; Sethi, G. Molecular targets of celastrol derived from Thunder of God Vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 2011, 303, 9–20. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.M.; Álvarez, X.A.; Vigo, C. Central nervous system effects of celastrol, a potent antioxidant and antiinflammatory agent. CNS Drug Rev. 2000, 6, 45–62. [Google Scholar] [CrossRef]
- Der Sarkissian, S.; Cailhier, J.; Borie, M.; Mansour, S.; Hamet, P.; Stevens, L.; Noiseux, N. Celastrol as a novel cardioprotective drug. Can. J. Cardiol. 2013, 29, S331. [Google Scholar] [CrossRef]
- Yang, G.; Wang, K.; Song, H.; Zhu, R.; Ding, S.; Yang, H.; Sun, J.; Wen, X.; Sun, L. Celastrol ameliorates osteoarthritis via regulating TLR2/NF-κB signaling pathway. Front. Pharmacol. 2022, 13, 963506. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.W.; Law, B.Y.K.; Mok, S.W.F.; Leung, E.L.H.; Fan, X.X.; Coghi, P.S.; Zeng, W.; Leung, C.H.; Ma, D.L.; Liu, L.; et al. Autophagic degradation of epidermal growth factor receptor in gefitinib-resistant lung cancer by celastrol. Int. J. Oncol. 2016, 49, 1576–1588. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Yu, P.; Jiang, J.; Zhang, G.; Wang, Y. Synthesis and preliminary evaluation of neuroprotection of celastrol analogues in PC12 cells. Bioorganic Med. Chem. Lett. 2010, 20, 3844–3847. [Google Scholar] [CrossRef]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef]
- Jin, H.Z.; Hwang, B.Y.; Kim, H.S.; Lee, J.H.; Kim, Y.H.; Lee, J.J. Antiinflammatory constituents of celastrus o rbiculatus inhibit the NF-κB activation and NO production. J. Nat. Prod. 2002, 65, 89–91. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Yu, Y.; Zou, P.; Jiang, Y.; Sun, D. Characterization of celastrol to inhibit hsp90 and cdc37 interaction. J. Biol. Chem. 2009, 284, 35381–35389. [Google Scholar] [CrossRef]
- Wong, V.K.W.; Qiu, C.; Xu, S.W.; Law, B.Y.K.; Zeng, W.; Wang, H.; Michelangeli, F.; Dias, I.R.D.S.R.; Qu, Y.Q.; Chan, T.W. Ca2+ signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. Br. J. Pharmacol. 2019, 176, 2922–2944. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Bathon, J.M.; Emery, P. Rheumatoid arthritis. Lancet 2023, 402, 2019–2033. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Firestein, G.S. Rheumatoid arthritis—Common origins, divergent mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Liang, H.Y.; Yin, H.X.; Li, S.F.; Chen, Y.; Zhao, Y.J.; Hu, W.; Zhou, R.P. Calcium-permeable channels cooperation for rheumatoid arthritis: Therapeutic opportunities. Biomolecules 2022, 12, 1383. [Google Scholar] [CrossRef]
- Wong, V.K.; Li, T.; Law, B.Y.; Ma, E.D.; Yip, N.; Michelangeli, F.; Law, C.K.; Zhang, M.; Lam, K.Y.; Chan, P. Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis. 2013, 4, e720. [Google Scholar] [CrossRef]
- Xu, S.W.; Law, B.Y.K.; Qu, S.L.Q.; Hamdoun, S.; Chen, J.; Zhang, W.; Guo, J.R.; Wu, A.G.; Mok, S.W.F.; Zhang, D.W. SERCA and P-glycoprotein inhibition and ATP depletion are necessary for celastrol-induced autophagic cell death and collateral sensitivity in multidrug-resistant tumor cells. Pharmacol. Res. 2020, 153, 104660. [Google Scholar] [CrossRef] [PubMed]
- Coghi, P.; Ng, J.P.; Kadioglu, O.; Law, B.Y.K.; Qiu, A.C.; Saeed, M.E.; Chen, X.; Ip, C.K.; Efferth, T.; Liu, L. Synthesis, computational docking and biological evaluation of celastrol derivatives as dual inhibitors of SERCA and P-glycoprotein in cancer therapy. Eur. J. Med. Chem. 2021, 224, 113676. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kuan, H.; Wei, Q.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. (2R, 4aS, 6aS, 12bR, 14aS, 14bR) 10-Hydroxy-N-(4-((6-methoxyquinolin-8-yl) amino) pentyl)-2, 4a, 6a, 9, 12b, 14a-hexamethyl-11-oxo-1, 2, 3, 4, 4a, 5, 6, 6a, 11, 12b, 13, 14, 14a, 14b-tetradecahydropicene-2-carboxamide. Molbank 2023, 2023, M1716. [Google Scholar] [CrossRef]
- Klaić, L.; Morimoto, R.I.; Silverman, R.B. Celastrol analogues as inducers of the heat shock response. Design and synthesis of affinity probes for the identification of protein targets. ACS Chem. Biol. 2012, 7, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Lopes, J.R.; Dos Santos, J.L.; Scarim, C.B. Phthalimide as a versatile pharmacophore scaffold: Unlocking its diverse biological activities. Drug Dev. Res. 2023, 84, 1346–1375. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Utreja, D. A Review on Drug Discovery of Phthalimide Analogues as Emerging Pharmacophores: Synthesis and Biological Potential. ChemistrySelect 2025, 10, e202405580. [Google Scholar] [CrossRef]
- Aljuhani, A.; Nafie, M.S.; Albujuq, N.R.; Hourani, W.; Albelwi, F.F.; Darwish, K.M.; Samir Ayed, A.; Reda Aouad, M.; Rezki, N. Unveiling the anti-cancer potentiality of phthalimide-based Analogues targeting tubulin polymerization in MCF-7 cancerous Cells: Rational design, chemical Synthesis, and Biological-coupled Computational investigation. Bioorganic Chem. 2024, 153, 107827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Martin, K.K.; Stewart, A.K. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk. Lymphoma 2013, 54, 683–687. [Google Scholar] [CrossRef]
- Santos, J.L.; Yamasaki, P.R.; Chin, C.M.; Takashi, C.H.; Pavan, F.R.; Leite, C.Q. Synthesis and in vitro anti Mycobacterium tuberculosis activity of a series of phthalimide derivatives. Bioorg Med. Chem. 2009, 17, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Upadhyay, C.; Poonam; Kumar, S.; Rathi, B. Phthalimide analogs for antimalarial drug discovery. RSC Med. Chem. 2021, 12, 1854–1867. [Google Scholar] [CrossRef]
- Khan, M.; Aatasam Hanif, M.; Rehman, K.; Arif, M.; Nazir, H.; Wali Khan, S.; Kangal, A.; Abid, R.; Dunia, A.A.F.; Mohamed, S.E.; et al. Molecular docking and in vitro antibacterial activity of chiral phthalimide on ESBL producing gram negative bacteria. Pak. J. Pharm. Sci. 2023, 36, 681–697. [Google Scholar] [PubMed]
- Heras Martinez, H.M.; Barragan, E.; Marichev, K.O.; Chávez-Flores, D.; Bugarin, A. Phthalimides as anti-inflammatory agents. Future Med. Chem. 2025, 17, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wen, J.; Hua, Y.; Zhu, Y.; Xia, Q.; Guo, Q.; Luo, Y.; Deng, X.; Huang, Y. Synthesis and anticancer properties of celastrol derivatives involved in the inhibition of VEGF. J. Enzym. Inhib. Med. Chem. 2023, 38, 2238137. [Google Scholar] [CrossRef]
- Yuzhu, G.; Anyanwu, M.; Yang, X.; Zimo, R.; Gianoncelli, A.; Ribaudo, G.; Coghi, P. (2R,4aS,6aS,12bR,14aS,14bR)-N-(2-(2-(2-(2-Azidoethoxy)ethoxy)ethoxy)ethyl)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxamide. Molbank 2024, 2, M1800. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Z.; Ma, Z.; Li, M.; Du, L. Astemizole Derivatives as Fluorescent Probes for hERG Potassium Channel Imaging. ACS Med. Chem. Lett. 2016, 7, 245–249. [Google Scholar] [CrossRef]
- Jiang, A.; Anyanwu, M.; Leong, K.; Li, J.; Gianoncelli, A.; Coghi, P.; Ribaudo, G. 3-[(1H-Benzo[d][1,2,3]triazol-1-yl)oxy]propyl 9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylate. Molbank 2022, 3, M1419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).