Abstract
A new π-conjugated acceptor–donor–acceptor small molecule, designed for applications in organic solar cells, containing a terthiophene core and indandione- and benzonitrile-based electron-withdrawing units, was synthesized via a multi-step process involving Suzuki–Miyaura cross-coupling and Knoevenagel condensation reactions. The structure was confirmed by NMR spectroscopy, HRMS, and its optoelectronic properties were evaluated by UV–vis spectroscopy and cyclic voltammetry.
1. Introduction
Solar energy stands out as one of the most promising alternatives to conventional fossil fuels due to its abundance and minimal environmental impact [1,2]. Among the various solar technologies, organic solar cells (OSCs) have gained significant attention for their lightweight nature, mechanical flexibility, and low fabrication costs. However, despite notable improvements in recent years, OSCs continue to lag behind traditional silicon-based solar cells, particularly in terms of power conversion efficiency (PCE) and long-term operational stability [2,3,4]. To overcome these limitations, the development of advanced and innovative materials remains essential for pushing the performance boundaries of OSCs. The acceptor–donor–acceptor (A–D–A) molecular architecture has emerged as a successful approach in the development of high-performance materials. Electron-deficient acceptor units facilitate efficient charge separation and enhance light-harvesting capabilities, while the donor unit governs the charge transport properties and optical behavior of the molecule. The choice and combination of donor and acceptor units play a pivotal role in determining both the efficiency and long-term stability of the final material [4,5].
A promising candidate as a donor unit is terthiophene, a π-conjugated molecule composed of three thiophene rings bonded together [6,7,8]. The extended conjugation of terthiophene allows for efficient charge transport and a broad absorption spectrum, especially in the visible range. Furthermore, its electron-rich nature makes it an ideal donor material that can facilitate efficient hole transfer in OSC devices [7,8]. Terthiophene-based materials have been widely studied for their robust electronic properties, which contribute to improved performance in organic electronics and photovoltaics [7,8,9,10].
On the other hand, the acceptor unit plays a crucial role in enhancing the material’s ability to separate and transport charge carriers. Indandione derivatives, known for their electron-deficient character, represent an excellent option for the acceptor units in OSCs. The conjugated structure of indandione, which includes carbonyl groups, provides strong electron-withdrawing properties that lower the LUMO energy levels and promote efficient electron transport. This contributes to a more effective charge separation mechanism, essential for optimizing device performance [11,12,13]. Moreover, the absorption capabilities of indandione derivatives in the near-infrared region further enhance the photon harvesting potential of the material.
Building upon the foundational work of Jean Roncali and our research group [14,15,16,17,18], this study introduces a new A–D–A small molecule, incorporating terthiophene as a donor unit and benzonitrile and indandione derivatives as acceptor units. The goal is to engineer an active-layer material with optimized optoelectronic properties that is capable of improving both PCE and operational stability in OSCs. In this article, we detail the synthesis and characterization of such a molecule, highlighting its potential for contributing to the next generation of high-performance low-cost organic photovoltaic devices.
2. Results and Discussion
The synthesis of the target compound 11 was accomplished through a multi-step reaction pathway outlined in Scheme 1. Intermediates 2 [19], 5 [19], 7 [19], 8 [20], and 10 [13] were prepared according to previously reported methods. In contrast to previous work [19] in which compounds 4 and 6 were synthesized via Stille coupling, in this study, they were successfully obtained using the Suzuki–Miyaura cross-coupling reaction, which afforded good yields, such as 70% and 53%, respectively.
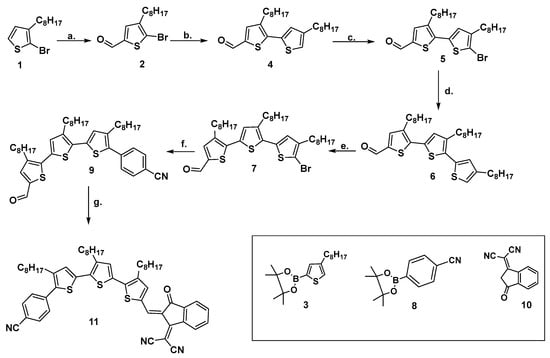
Scheme 1.
Synthetic strategy for the access to the target donor–acceptor compound 11: (a) POCl3, DMF, 1,2-dichloroethane, 3 h, 60 °C, 37%; (b) Compound 3, Dioxane/H2O, Na2CO3; Pd(dppf)Cl2, 18 h, reflux, 53%; (c) N-Bromosuccinimide, CHCl3, CH3COOH, 24 h, 25 °C, 43%; (d) Compound 3, Dioxane/H2O, Na2CO3; Pd(dppf)Cl2, 18 h, reflux, 71%; (e) N-Bromosuccinimide, CHCl3, CH3COOH, 24 h, 25 °C, 53%; (f) Compound 8, Dioxane/H2O, Na2CO3; Pd(dppf)Cl2, 16 h, reflux, 77%; (g) Compound 10, pyridine, dry toluene, 4 h, 65 °C, 89%.
The synthetic route toward the target compound 11 started with the Vilsmeier–Haack formylation of 2-bromo-3-octylthiophene 1. This reaction selectively introduced an aldehyde group onto the thiophene ring, yielding the formylated derivative 2. Subsequent steps involved a sequence of Suzuki–Miyaura cross-coupling reaction combined with regioselective bromination steps. These transformations were strategically employed to construct a highly conjugated thiophene-based π-system, thereby facilitating the formation of intermediate 7. Subsequent coupling of derivative 7 with a homemade 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzonitrile 8, prepared from 4-bromobenzonitrile and bis(pinacolato)diboron using a standard protocol [20] via Suzuki–Miyaura reaction, yielded compound 9. Finally, a Knoevenagel condensation between intermediate 9 and 2-(3-oxo-2,3-dihydro-1H-inden-1-ylidene) malononitrile 10 yielded the target compound 11.
To evaluate the viability of compound 11 for application in the active layers of organic solar cells, a comprehensive analysis of its optical and electrochemical properties was conducted. Figure 1a shows the normalized UV–vis absorption spectra of compound 11 in four different solvents: chloroform (CHCl3), acetone, tetrahydrofuran (THF), and toluene. A clear solvatochromic effect is noticed, demonstrated by the gradual bathochromic shift in the absorption maximum from 558 nm to 592 nm across the solvent series (Table 1). The observed red shift indicates enhanced stabilization of the excited state in a polar environment, consistent with an intramolecular charge transfer (ICT) character of the electronic transition. Figure 1b compares the normalized UV–vis absorption spectra of compound 11 in chloroform solution and as a thin film deposited on a glass substrate from the same solvent. In the solid state, the absorption maximum shifts to a shorter wavelength, from approximately 592 nm in CHCl3 to around 566 nm in the film, suggesting the formation of H-aggregates. Similar behavior has been previously observed in other thiophene-based oligomers [15]. The broadened absorption profile observed in the film arises from intermolecular interactions in the solid state and potential aggregate formation, factors that may improve the light-harvesting performance of organic photovoltaic systems. The optical bandgap, determined from the absorption edge of the thin film, is estimated to be 1.70 eV.
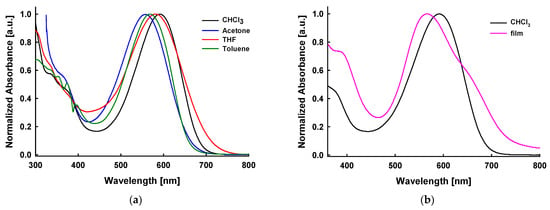
Figure 1.
(a) UV–vis absorption spectra of compound 11 in various solvents (10−5 M); (b) normalized absorption spectra in solution (black line) and thin film spin-coated on glass (purple line) of the compound 11.

Table 1.
Optical and electrochemical data for target compound 11 s: solution; f: thin film on glass; a: estimated from the long-wavelength absorption onset of thin films; b: estimated from the onset of the oxidation and reduction processes; c: bandgap calculated from the difference between HOMO–LUMO levels.
The electrochemical properties of derivative 11 have been analyzed by cyclic voltammetry in dichloromethane solutions in the presence of tetrabutylammonium hexafluorophosphate as supporting electrolyte. Figure 2 shows the cyclic voltammograms (CVs) of compound 11. The CV of derivative 11 shows two quasi-reversible oxidation waves Epa1 and Epa2 at 1.15 and 1.48 V, corresponding to the formation of a cation radical and dication, respectively. In the negative region, the CV of the compound 11 displays an irreversible reduction process with cathodic peak potential (Epc) at −0.77 V, indicative of an unstable anion radical. Moreover, the HOMO (approximately −5.68 eV; Eonsetox = 1.00 V vs. SCE) and LUMO (approximately −4.03 eV; Eonsetred = −0.65 V vs. SCE) energy levels, derived from the onset potentials of the first oxidation and reduction waves, are well aligned with the electronic requirements encountered in performant donors in bulk heterojunction solar cells with non-fullerene acceptors such as PC61BM (EHOMO between −5.2 and −5.8 eV; ELUMO between −3.7 and −4.0 eV) [21,22,23]. Notably, the electrochemical bandgap (1.65 eV) closely matches the optical bandgap (1.70 eV) derived from UV–vis spectroscopy. Thus, our next step will be testing them as a donor in bulk heterojunction solar cells.
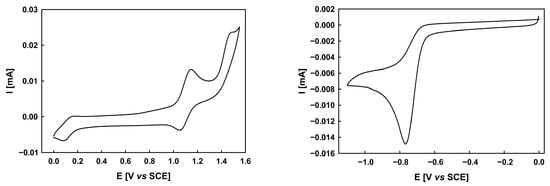
Figure 2.
Cyclic voltammograms corresponding to the oxidation (left) and reduction (right) of the target compound 11 in 0.10 M Bu4NPF6/CH2Cl2, scan rate 100 mVs−1, Pt electrode.
3. Materials and Methods
3.1. General Experimental Details
All the commercial reagents and chemicals were used without purification. Solvents were dried using standard techniques. Thin-layer chromatography was performed on silica gel 60 F254 with detection by UV irradiation at 254 nm and 365 nm. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE III 600 (Bruker Daltonics, Bremen, Germany) operating at 600 MHz for 1H and 150 MHz for 13C. The solvent used was deuterated chloroform (CDCl3), and the chemical shifts are reported in parts per million (ppm) using the residual solvent peak as internal reference. UV–vis measurements were performed in various solvents (HPLC-grade) at room temperature using a Shimadzu UV spectrophotometer (UV-1800) (Shimadzu Corporation, Kyoto, Japan). Electrochemical experiments were performed with a BioLogic SP-150 Potentiostat (BioLogic, Seyssinet-Pariset, France) in a standard three-electrode cell using platinum electrodes and a saturated calomel reference electrode (SCE).
3.2. Synthetic Procedures
3.2.1. Synthesis of 3,4′-Dioctyl-[2,2′-bithiophene]-5-carbaldehyde (4)
Compound 2 (0.8 g, 2.63 mmol), Na2CO3 (1.11 g, 10.55 mmol), and 4,4,5,5-tetramethyl-2-(4-octylthiophen-2-yl)-1,3,2-dioxaborolane (compound 3) (1.1 g, 3.42 mmol) were dissolved in a mixture of dioxane (80 mL)/water (5 mL) and degassed with argon for 30 min at room temperature. Then, Pd(dppf)Cl2 (0.19 g, 0.263 mmol) was added, and the mixture was refluxed overnight. After cooling to room temperature, the reaction mixture was extracted with dichloromethane (3 × 10 mL), and the combined organic layers were washed with water and brine, then dried over MgSO4. Solvents were removed under low pressure. Purification was conducted by column chromatography on silica gel using 1:30 ethyl acetate: petroleum ether as eluent to afford a yellow oil (592 mg, 1.36 mmol, 53% yield).
1H NMR (600 MHz, Chloroform-d) δ (ppm): 9.82 (s, 1H), 7.58 (s, 1H), 7.11 (s, 1H), 7.01 (s, 1H), 2.78 (t, J = 7.8 Hz, 2H), 2.61 (t, J = 7.6 Hz, 2H), 1.68–1.61 (overlapped signals, 4H), 1.39–1.23 (overlapped signals, 20H), 0.89–0.87 (overlapped signals, 6H), see 1H NMR spectrum in Supplementary Materials.
3.2.2. Synthesis of 3,4′,4″-Trioctyl-[2,2′,2″-terthiophene]-5-carbaldehyde (6)
A mixture of compound 5 (0.236 g, 0.476 mmol), Na2CO3 (0.200 g, 1.90 mmol), and compound 3 (0.200 g, 0.620 mmol) was dissolved in a dioxane (40 mL)/water (5 mL) mixture and degassed with argon for 30 min at room temperature. Subsequently, Pd(dppf)Cl2 (0.034 g, 0.0476 mmol) was added, and the reaction mixture was stirred under reflux overnight. Upon completion, the reaction was allowed to cool to room temperature and extracted with dichloromethane (3 × 5 mL). The combined organic layers were washed sequentially with water and brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel using a 1:30 mixture of ethyl acetate and petroleum ether as the eluent, yielding a dark yellow oil (206 mg, 0.330 mmol) in 70% yield. 1H NMR (600 MHz, Chloroform-d) δ (ppm): 9.82 (s, 1H), 7.58 (s, 1H), 7.11 (s, 1H), 6.99 (s, 1H), 6.94 (s, 1H), 2.81 (t, J = 7.7 Hz, 2H), 2.75 (t, J = 7.8 Hz, 2H), 2.61 (t, J = 7.7 Hz, 2H), 1.71–1.61 (overlapped signals, 6H), 1.43–1.27 (overlapped signals, 30H), 0.91–0.81 (overlapped signals, 9H).
3.2.3. Synthesis of 4-(5″-Formyl-3′,3″,4-trioctyl-[2,2′:5′,2″-terthiophen]-5-yl)benzonitrile (9)
Adapted after a previously reported procedure [20]: compound 7 (0.1 g, 0.14 mmol), Na2CO3 (0.059 g, 0.56 mmol), and compound 8 (0.043 g, 0.182 mmol) were dissolved in a mixture of dioxane (8 mL) and water (2 mL) and degassed with argon for 30 min at room temperature. Subsequently, Pd(dppf)Cl2 (0.010 g, 0.014 mmol) was added, and the reaction mixture was refluxed overnight. After cooling at room temperature, the mixture was extracted with dichloromethane (3 × 5 mL), and the combined organic layers were washed with water and brine, then dried over anhydrous MgSO4. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel using 1:50 dichloromethane: petroleum ether as eluent (Rf 0.38), affording a yellow solid (76 mg, 0.107 mmol, 77% yield).
1H NMR (600 MHz, Chloroform-d) δ (ppm): 9.83 (s, 1H), 7.71(d, J = 8.2 Hz, 2H), 7.59 (s, 1H), 7.57 (d, J = 8.2 Hz, 2H), 7.13 (s, 1H), 7.07 (s, 1H), 2.81–2.76 (overlapped signals, 4H), 2.67 (t, J = 7.9 Hz, 2H), 1.69–1.59 (overlapped signals, 6H), 1.39–1.26 (overlapped signals, 30H), 0.89–0.86 (overlapped signals, 9H). HRMS (APCI+): m/z [M + H]+ calc for C44H60NOS3: 714.38315; found: 714.38525, see APCI (+)-HRMS spectrum in Supplementary Materials.
3.2.4. Synthesis of 2-(2-((5″-(4-Cyanophenyl)-3,4′,4″-trioctyl-[2,2′:5′,2″-terthiophen]-5-yl)methylene)-3-oxo-2,3-dihydro-1H-inden-1-ylidene) Malononitrile (11)
Adapted from a literature procedure [12]: a solution of compound 9 (0.070 g, 0.098 mmol) and compound 10 (0.028 g, 0.147 mmol) in 5 mL of dry toluene with 0.1 mL of pyridine was stirred at 65 °C. Upon completion of the reaction, as monitored by TLC, the solvent was removed under vacuum, and the solid was washed several times with ethanol to yield a deep blue solid, m.p. 133–136 °C (78 mg, 0.088 mmol, 89% yield).
1H NMR (600 MHz, Chloroform-d) δ (ppm): 8.81 (s, 1H), 8.70 (d, J = 7.6 Hz, 1H), 7.94 (d, J = 7.2 Hz, 1H), 7.80–7.74 (overlapped signals, 3H), 7.72 (d, J = 7.6 Hz, 2H), 7.66 (s, 1H), 7.58 (d, J = 7.6 Hz, 2H), 7.38 (s, 1H), 7.12 (s, 1H), 2.87–2.83 (overlapped signals, 4H), 2.69 (t, J = 8.0 Hz, 2H), 1.81–1.60 (overlapped signals, 6H), 1.46–1.26 (overlapped signals, 30H), 0.88–0.86 (overlapped signals, 9H), see 1H NMR spectrum in Supplementary Materials. 13C NMR (150 MHz, Chloroform-d) δ (ppm): 188.46, 160.52, 148.43, 148.12, 141.08, 141.01, 140.99, 140.06, 138.94, 137.55, 136.93, 136.34, 135.19, 135.10, 134.54, 134.36, 134.17, 133.00, 132.44, 131.46, 129.49, 129.24, 125.36, 123.77, 122.40, 118.77, 118.76, 114.62, 114.59, 110.94, 69.50, 69.46, 31.90, 31.87, 31.86, 30.82, 30.55, 30.03, 29.71, 29.61, 29.61, 29.52, 29.48, 29.46, 29.42, 29.37, 29.35, 29.34, 29.28, 29.26, 29.23, 29.21, 28.90, 28.66, 22.67, 14.11, see 13C NMR spectrum in Supplementary Materials. HRMS (APCI+): m/z [M + H]+ calc for C56H64N3OS3: 890.42060; found: 890.41620, see APCI (+)-HRMS spectrum in Supplementary Materials.
4. Conclusions
This study presents the synthesis of a novel compound with promising potential as an active-layer material in organic solar cells. The structure of the compound was elucidated and confirmed through comprehensive characterization techniques, including 1H and 13C nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS), cyclic voltammetry, and ultraviolet–visible (UV–vis) spectroscopy. Optical and electrochemical analyses revealed broad absorption in the visible region and redox behavior, indicative of the efficient intramolecular charge transfer required in OSCs’ active materials.
Supplementary Materials
Figure S1. 1H NMR spectrum of compound 3. Figure S2. 1H NMR spectrum of compound 4. Figure S3. 1H NMR spectrum of compound 5. Figure S4. 1H NMR spectrum of compound 6. Figure S5. 1H NMR spectrum of compound 7. Figure S6. 1H NMR spectrum of compound 8. Figure S7. 1H NMR spectrum of compound 9. Figure S8. APCI (+)-HRMS spectrum of compound 9. Figure S9. 1H NMR spectrum of compound 10. Figure S10. 1H NMR spectrum of compound 11. Figure S11. 13C NMR spectrum of compound 11. Figure S12. APCI (+)-HRMS spectrum of compound 11.
Author Contributions
The conception and design of this study were contributed by A.T. and I.G., A.M.F., D.B. and L.C. performed the synthesis, purification, and characterization of the compounds. A.M.F. and A.P.C. performed the optical and electrochemical measurements. A.P.C. and I.G. wrote the sections of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Romanian Ministry of Research, Innovation and Digitization, CNCS—UEFISCDI, research project PN-III-P4-ID-PCE-2021-1812 (ICOFCOSC).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| OSCs | Organic Solar Cells |
| A–D–A | Acceptor–Donor–Acceptor |
| PCE | Power Conversion Efficiency |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| UV–vis | Ultraviolet–visible |
| CV | Cyclic Voltammetry |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| HPLC | High-Performance Liquid Chromatography |
| ICT | Intramolecular Charge Transfer |
| PC61BM | Phenyl-C61-butyric acid methyl ester |
| dppf | 1,1′-Bis(diphenylphosphino)ferrocene |
| THF | Tetrahydrofuran |
References
- Yi, J.; Zhang, G.W.; Yu, H.; He, Y. Advantages, Challenges and Molecular Design of Different Material Types Used in Organic Solar Cells. Nat. Rev. Mater. 2024, 9, 46–62. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the Planet: Chemical Challenges in Solar Energy Utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, R.; Yang, Y. Polymer Solar Cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.G. An electron acceptor challenging fullerene for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y. Development of novel conjugated donor polymers for high-efficiency bulk-heterojunction photovoltaic devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Colella, N.S.; Cherniawski, B.P.; Mannsfeld, S.C.B.; Briseno, A.L. Oligothiophene Semiconductors: Synthesis, Characterization, and Applications for Organic Devices. ACS Appl. Mater. Interfaces 2014, 6, 5327–5343. [Google Scholar] [CrossRef] [PubMed]
- Farka, D.; Ciganek, M.; Veselý, D.; Kalina, L.; Krajčovič, J. Epitaxial Guidance of Adamantyl-Substituted Polythiophenes by Self-Assembled Monolayers. ACS Omega 2024, 9, 38733–38742. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Fréchet, J.M.J. Molecular design and ordering effects in π-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 2011, 133, 20009–20029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, D.; Xu, Y.; Feng, X. Thiophene-based conjugated oligomers for organic solar cells. J. Mater. Chem. 2011, 21, 17590–17600. [Google Scholar] [CrossRef]
- Chen, L.; Wu, X.; Li, Z.; Niu, R.; Zhou, W.; Liu, K.; Sun, Y.; Shao, Z.; Yang, J.; Song, Y. D–π–A type conjugated indandione derivatives: Ultrafast broadband nonlinear absorption responses and transient dynamic. RSC Adv. 2022, 12, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- Terenti, N.; Giurgi, G.-I.; Szolga, L.; Stroia, I.; Terec, A.; Grosu, I.; Crișan, A.P. Effect of the Terminal Acceptor Unit on the Performance of Non-Fullerene Indacenodithiophene Acceptors in Organic Solar Cells. Molecules 2022, 27, 1229. [Google Scholar] [CrossRef] [PubMed]
- Terenti, N.; Giurgi, G.-I.; Crişan, A.P.; Anghel, C.; Bogdan, A.; Pop, A.; Stroia, I.; Terec, A.; Szolga, L.; Grosu, I.; et al. Structure–properties of small donor–acceptor molecules for homojunction single-material organic solar cells. J. Mater. Chem. C 2022, 10, 5716–5726. [Google Scholar] [CrossRef]
- Roncali, J. Synthetic principles for band gap control in linear π-conjugated systems. Chem. Rev. 1997, 97, 173–206. [Google Scholar] [CrossRef] [PubMed]
- Roncali, J.; Frère, P.; Blanchard, P.; de Bettignies, R.; Turbiez, M.; Roquet, S.; Leriche, P.; Nicolas, Y. Molecular and supramolecular engineering of k-conjugated systems for photovoltaic conversion. Thin Solid Film. 2006, 511, 567–575. [Google Scholar] [CrossRef]
- Demeter, D.; Allain, M.; Leriche, P.; Grosu, I.; Roncali, J. Synthesis and Electronic Properties of Terthienyls β-Substituted by (Thienyl)Cyanovinylene Groups. Tetrahedron Lett. 2010, 51, 4117–4120. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P. Molecular materials for organic photovoltaics: Small molecules versus polymers. Acc. Chem. Res. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Roncali, J.; Grosu, I. The Dawn of Single Material Organic Solar Cells. Adv. Sci. 2019, 6, 1801026. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, X.; Wang, F.; Zhou, J.; Long, G.; Tian, J.; Chen, Y. High-Performance Solar Cells using a Solution-Processed Small Molecule Containing Benzodithiophene Unit. Adv. Mater. 2011, 23, 5387–5391. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wu, Y.; Cheung, M.-S.; Ho, C.-L.; Dong, Q.; Lin, Z.; Li, Y.; Wong, W.-Y. Design, synthesis and in vitro and in vivo antitumour activity of 3-benzylideneindolin-2-one derivatives, a novel class of small-molecule inhibitors of the MDM2-p53 interaction. Eur. J. Med. Chem. 2014, 81, 277–288. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design Rules for Donors in Bulk-Heterojunction Solar Cells—Towards 10 % Energy-Conversion Efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Mihailetchi, V.D.; Blom, P.W.M. Ultimate efficiency of polymer/fullerene bulk heterojunction solar cells. Appl. Phys. Lett. 2006, 88, 093511. [Google Scholar] [CrossRef]
- Thompson, B.C.; Kim, Y.-G.; Reynolds, J.R. Spectral Broadening in MEH-PPV:PCBM-Based Photovoltaic Devices via Blending with a Narrow Band Gap Cyanovinylene−Dioxythiophene Polymer. Macromolecules 2005, 38, 5359–5362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).