4-([1,1′:3′,1′′-terphenyl]-2′-yloxy)-5-chlorophthalonitrile
Abstract
1. Introduction
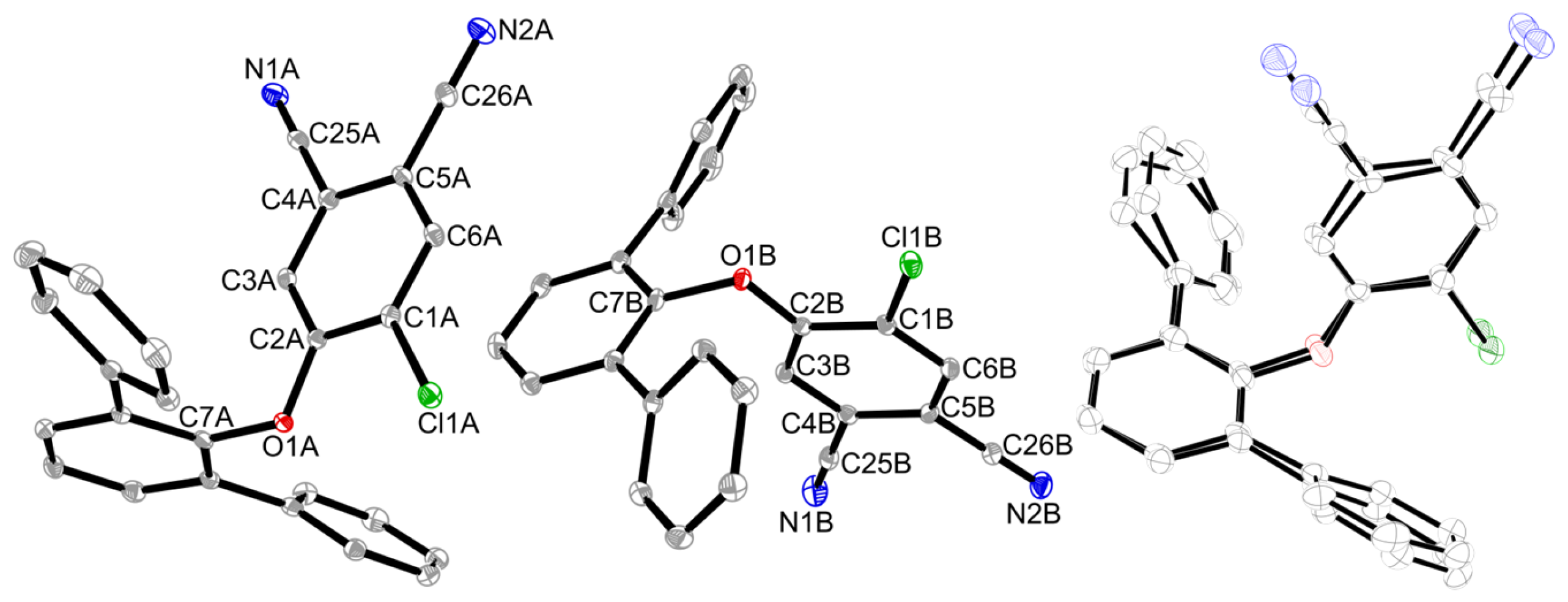
2. Results
3. Materials and Methods
3.1. Synthesis of 4-([1,1′:3′,1″-terphenyl]-2′-yloxy)-5-chlorophthalonitrile
3.2. X-Ray Crystallography
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erzunov, D.; Rassolova, A.; Rumyantsev, R.; Maizlish, V.; Tikhomirova, T.; Vashurin, A. Crystal Structures of 4-(2/3-Methoxyphenoxy)-Phthalonitrile. Acta Crystallogr. E Crystallogr. Commun. 2023, 79, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Derradji, M.; Wang, J.; Liu, W.B. High Performance Ceramic-Based Phthalonitrile Micro and Nanocomposites. Mater. Lett. 2016, 182, 380–385. [Google Scholar] [CrossRef]
- Köç, M.; Zorlu, Y.; Işci, Ü.; Berber, S.; Ahsen, V.; Dumoulin, F. A Library of Dimeric and Trimeric Phthalonitriles Linked by a Single Aromatic Ring: Comparative Structural and DFT Investigations. CrystEngComm 2016, 18, 1416–1426. [Google Scholar] [CrossRef]
- Keller, T.M. Imide-Containing Phthalonitrile Resin. Polymer (Guildf) 1993, 34, 952–955. [Google Scholar] [CrossRef]
- Erzunov, D.; Rassolova, A.; Botnar, A.; Tonkova, S.; Rumyantsev, R.; Maizlish, V.; Aleksandriskii, V.; Vashurin, A. The Influence of Methoxy- Group Position on Thermal Stability and Properties of Novel Isomeric 4-[(Methoxy)Phenoxy] Phthalonitriles and Phthalocyanine Complexes Based on Them. Dye. Pigment. 2023, 219, 111600. [Google Scholar] [CrossRef]
- Anderson, D.R.; Solntsev, P.V.; Rhoda, H.M.; Nemykin, V.N. How big is big? Separation by conventional methods, X-ray and electronic structures of positional isomers of bis-tertbutylisocyano adduct of 2(3),9(10),16(17),23(24) tetrachloro3(2),10(9),17(16),24(23)-tetra(2,6-di-iso-propylphenoxy)-phthalocyaninato iron(II) complex. J. Porphyr. Phthalocyanines 2016, 20, 337–351. [Google Scholar] [CrossRef]
- Yazici, S.; Akdemir, N.; Agar, E.; Kantar, C.; Senel, I. 4-Chloro-3-(2,3,5-trimethylphenoxy)phthalonitrile. Acta Crystallogr. E Struct. Rep. 2005, E61, o782–o783. [Google Scholar] [CrossRef]
- Husain, A.; Ganesan, A.; Machacek, M.; Cerveny, L.; Kubat, P.; Ghazal, G.; Zimcik, P.; Makhseed, S. Dually Directional Glycosylated Phthalocyanines as Extracellular Red-Emitting Fluorescent Probes. Dalton Trans. 2020, 49, 9605–9617. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Inoue, Y.; Yuge, H. The effect of Clp interactions on the conformations of 4-chloro5-(2-phenoxyethoxy)phthalonitrile and 4-chloro-5-[2-(pentafluorophenoxy)ethoxy]phthalonitrile. Acta Crystallogr. C Cryst. Struct. Commun. 2011, C67, o154–o156. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A Critical Account on π-π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Rumyantsev, R.V.; Katkova, M.A.; Zabrodina, G.S.; Ketkov, S.Y. Features of Dicyanamide Binding to a Polynuclear Metallamacrocyclic Copper Complex. J. Struct. Chem. 2023, 64, 1635–1643. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorkii, P.M. New applications of van der Waals radii in chemistry. Rus. Chem. Rev. 1995, 64, 446. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Bondi, A. Van der waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Umezawa, Y.; Tsuboyama, S.; Takahashi, H.; Uzawa, J.; Nishio, M. CH/π interaction in the conformation of peptides. A database study. Bioorg. Med. Chem. 1999, 7, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Cl-π interactions in protein-ligand complexes. Protein Sci. 2008, 17, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Bankoti, N.; Michael, D.; Sekar, K.; Row, T.G. C-halogen… pi interactions in nucleic acids: A database study. J. Chem. Sci. 2020, 132, 93. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, ver. 1.171.42.72a; Rigaku Corporation: Oxford, UK, 2016; Volume 32, pp. 31–34. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]





| Bond | Molecule A | Molecule B | Angle | Molecule A | Molecule B |
|---|---|---|---|---|---|
| C(1)-Cl(1) | 1.7293(16) | 1.7225(16) | C(2)-O(1)-C(7) | 117.22(11) | 116.31(12) |
| O(1)-C(2) | 1.3579(18) | 1.3611(18) | C(2)-C(1)-Cl(1) | 119.23(12) | 119.36(12) |
| O(1)-C(7) | 1.4128(17) | 1.4163(17) | C(6)-C(1)-Cl(1) | 119.66(12) | 120.17(12) |
| N(1)-C(25) | 1.150(2) | 1.149(2) | O(1)-C(2)-C(1) | 116.23(13) | 116.57(13) |
| N(2)-C(26) | 1.147(2) | 1.151(2) | O(1)-C(2)-C(3) | 124.07(13) | 123.91(13) |
| C(1)-C(2) | 1.408(2) | 1.409(2) | N(1)-C(25)-C(4) | 179.77(18) | 179.9(3) |
| C(2)-C(3) | 1.393(2) | 1.391(2) | N(2)-C(26)-C(5) | 179.05(19) | 179.7(2) |
| C(3)-C(4) | 1.398(2) | 1.394(2) | C(3)-C(4)-C(25) | 120.07(14) | 119.77(14) |
| C(4)-C(5) | 1.412(2) | 1.408(2) | C(5)-C(4)-C(25) | 119.50(14) | 120.19(14) |
| C(5)-C(6) | 1.395(2) | 1.393(2) | C(4)-C(5)-C(26) | 119.76(14) | 119.61(15) |
| C(1)-C(6) | 1.382(2) | 1.387(2) | C(6)-C(5)-C(26) | 120.51(14) | 120.56(14) |
| C(4)-C(25) | 1.447(2) | 1.444(2) | N/A | N/A | N/A |
| C(5)-C(26) | 1.442(2) | 1.441(2) | N/A | N/A | N/A |
| D–H…A/D…A | D–H | H…A | D…A | D–H…A |
|---|---|---|---|---|
| C3A–H3AA…N1A i,* | 0.95 | 2.65 | 3.509(2) | 149.8 |
| C6A–H6AA…N2B ii | 0.95 | 2.53 | 3.352(2) | 144.3 |
| C6B–H6BA…N2A iii | 0.95 | 2.68 | 3.501(2) | 144.8 |
| N1A…C25A i | N/A | N/A | 3.451(2) | N/A |
| N2A…C26B ii | N/A | N/A | 3.343(2) | N/A |
| C26A…N2B ii | N/A | N/A | 3.280(2) | N/A |
| (C19B-C24B)center…(C19B-C24B)center iv | N/A | N/A | 3.70 | N/A |
| (C20A-C21A)center…(C14B-C15B)center v | N/A | N/A | 3.37 | N/A |
| (C5A-C6A)center…(C9B-C10B)center | N/A | N/A | 3.57 | N/A |
| Cl1A…(C13B-C18B)center | N/A | N/A | 3.89 | N/A |
| Cl1B…(C19A-C24A)center vi | N/A | N/A | 3.41 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzunov, D.; Baklagin, V.; Abramov, I.; Maizlish, V.; Rumyantsev, R.; Vashurin, A. 4-([1,1′:3′,1′′-terphenyl]-2′-yloxy)-5-chlorophthalonitrile. Molbank 2025, 2025, M2028. https://doi.org/10.3390/M2028
Erzunov D, Baklagin V, Abramov I, Maizlish V, Rumyantsev R, Vashurin A. 4-([1,1′:3′,1′′-terphenyl]-2′-yloxy)-5-chlorophthalonitrile. Molbank. 2025; 2025(3):M2028. https://doi.org/10.3390/M2028
Chicago/Turabian StyleErzunov, Dmitry, Vyacheslav Baklagin, Igor Abramov, Vladimir Maizlish, Roman Rumyantsev, and Arthur Vashurin. 2025. "4-([1,1′:3′,1′′-terphenyl]-2′-yloxy)-5-chlorophthalonitrile" Molbank 2025, no. 3: M2028. https://doi.org/10.3390/M2028
APA StyleErzunov, D., Baklagin, V., Abramov, I., Maizlish, V., Rumyantsev, R., & Vashurin, A. (2025). 4-([1,1′:3′,1′′-terphenyl]-2′-yloxy)-5-chlorophthalonitrile. Molbank, 2025(3), M2028. https://doi.org/10.3390/M2028







