Abstract
(−)-Patagonic acid (1) is a clerodane diterpene isolated from several plants from the Alismataceae, Asteraceae, Euphorbiaceae, Fabaceae, Lamiaceae, Salicaceae, Sapindaceae, and Velloziaceae families, and its biological potential as an inhibitor of butyrylcholinesterase (BChE) and acetylcholinesterase (AChE) and as an anti-inflammatory compound has been described. Furthermore, the enantiomer (+)-1 is also described in Fabaceae and Verbenaceae. A lack of formal studies about the absolute configuration (AC) determination of 1 is emphasized. Thus, the present manuscript describes the AC determination of patagonic acid (1). The chemical correlation of (−)-1 from (−)-hardwickiic acid (2) was achieved by a simplistic oxidative process. The specific rotation value and electronic circular dichroism (ECD) analysis allowed for the AC determination of (−)-1 as (5R,8R,9S,10R)-(−)-patagonic acid. ECD revealed a positive exciton chirality (EC) phenomenon in both (−)-1 and (−)-2, which is directly associated with their configuration and conformational preferences, which were assessed by DFT calculations at the B3LYP/DGDZVP level of theory. Since the NMR data of (+)-1 are fully coincident with those from its enantiomer studied herein, the chirality of (5S,8S,9R,10S)-(+)-patagonic acid could also be determined. These experimental conclusions deeply complement the literature related to clerodane compounds biosynthesized in several families of plants of scientific interest.
1. Introduction
Clerodane diterpenes are bicyclic natural derivatives containing a chemical structure that includes a decalin system and a six-carbon appendix, which are generally oxidized to a diene, furan, lactone, or hydroxyfuran moiety, thereby having chemical diversity that favors an interesting variety of biological activities including antialimentary, opioid receptor agonist, antiulcer, cytotoxic, anti-inflammatory, antiparasitic, antibacterial, and antitumor [1,2]. Around 1300 clerodane derivatives have been isolated from plants, where chiral variations at C5, C8, C9, and C10 are also reported as diversity factors. In this regard, about 75% of the isolated clerodanes present a trans fusion at the decalin portion (C5–C10), while the remaining 25% possess the cis fusion. Furthermore, when the stereochemistry at C8 and C9 varies, clerodanes can be divided into C5—C10:C8—C9 trans:cis, trans:trans, cis:cis, and cis:trans variants [3,4]. In consequence, the absolute configuration (AC) determination in this class of diterpenoids represents a major issue for complete structural elucidation.
(−)-Patagonic acid (1) was first isolated in 1988 from the aerial parts of Baccharis patagonica (Asteraceae). Its structure was proposed based on the IR, UV, and NMR data, thus providing a relative configuration [5]. After that, compound (−)-1 was isolated from Echinodorus macrophyllus [6], Grangea maderaspatana [7], Croton megistocarpus [8], Copaifera cearensis [9], Eperua leucantha [10], Callicarpa hypoleucophylla [11], Callicarpa integerrima [12], Ballota limbata [13], Otostegia persica [14], Casearia sylvestris [15], Dodonea viscosa [16], and Nanuza plicata [17]. On the other hand, the enantiomer (+)-patagonic acid (1) has been isolated from Sindora sumatrana [18,19,20] and from Duranta repens [21]. Interestingly, in these reports, only the relative configuration of 1 was determined.
It is good to mention that (−)-patagonic acid (1) possesses the capability to inhibit butyrylcholinesterase (BChE) [14] and acetylcholinesterase (AChE) [13,14] and to suppress superoxide anions and elastase release, conferring anti-inflammatory potential [11], while (+)-1 inhibits the P-glycoprotein in an adriamycin-resistant human breast cancer cell line (MCF-7/ADR) [19]. The chirality of (−)-1 and (+)-1 can now be directly associated with their biological potential. The chiroptical properties are important in the pharmaceutical sciences due to biological receptors which are enantiospecific, and different responses could be displayed by the two enantiomers [22,23]. Then, in the present work, the AC determination of (−)-patagonic acid (1) is described by a simplistic chemical correlation using (−)-hardwickiic acid (2) as the starting material and MCPBA as the oxidant agent. The physical and spectroscopic data of (−)-1 agreed with those described, while the specific rotation values and electronic circular dichroism (ECD) measurements supported the absolute configuration as (−)-(5R,8R,9S,10R)-patagonic acid. ECD revealed a positive exciton chirality (EC) phenomenon in both compounds [(−)-1 and (−)-2], which is justified by their conformational preferences, which were assessed by DFT calculations at the B3LYP/DGDZVP level of theory. The overall results also provide evidence to establish the AC of (+)-1 from Sindora sumatrana and Duranta repens as (5S,8S,9R,10S)-(+)-patagonic acid. The results deeply complement the literature related to clerodane compounds biosynthesized in several families of plants of extensive chemical and biological interest.
2. Results and Discussion
(−)-Hardwickiic acid (2) was isolated from Chromolaena pulchella as previously reported by our research group, whose AC was established by vibrational circular dichroism (VCD) studies using the analogue hautriwaic acid lactone [24]. These results were concordant with previous configurational studies for (−)-2 isolated from Hardwickia pinnata [25]. The 1H NMR spectra revealed the characteristic signals at δ 7.35 (t, J = 1.6 Hz, H-15), 7.21 (dd, J = 1.6, 0.9 Hz, H-16), and 6.26 (dd, J = 1.6, 0.9 Hz, H-14), assigned to the furan moiety (Figures S8 and S9). The 13C NMR spectrum (Figure S10) showed the signals from furan in the range of δ 142.9–111.1 and the tactical signals at δ 37.7 (C5), δ 36.4 (C8), δ 38.9 (C9), and δ 46.8 (C10), distinctive for the spatial configuration as depicted in Figure 1.
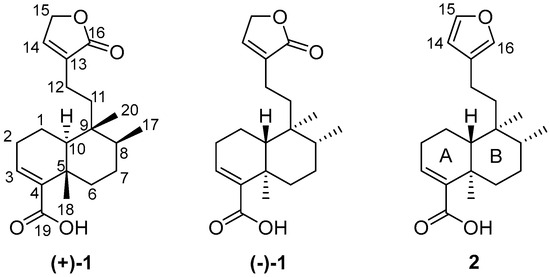
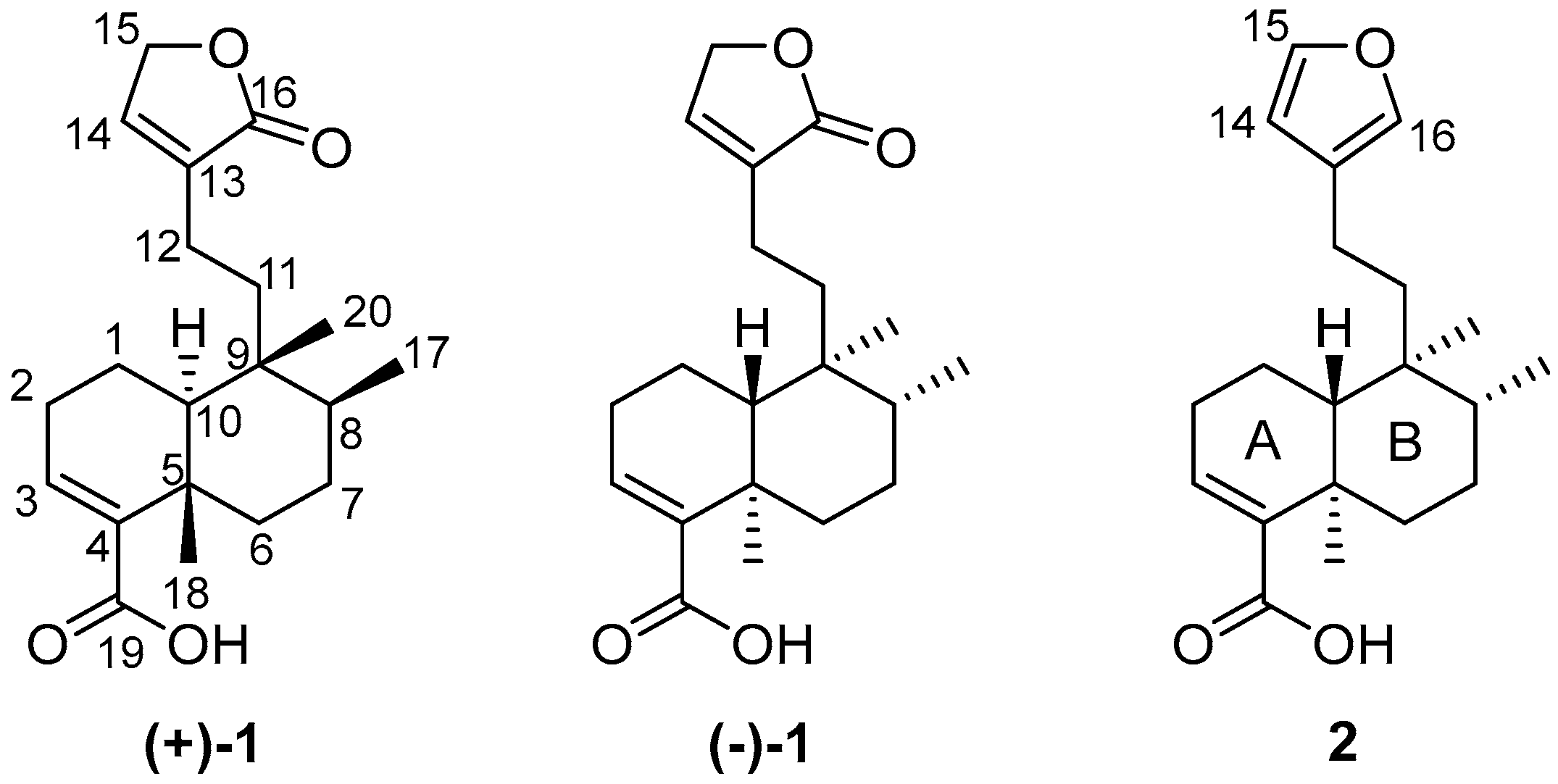
Figure 1.
Formulas of patagonic acids (+)-1 and (−)-1, and (−)-hardwickiic acid (2).
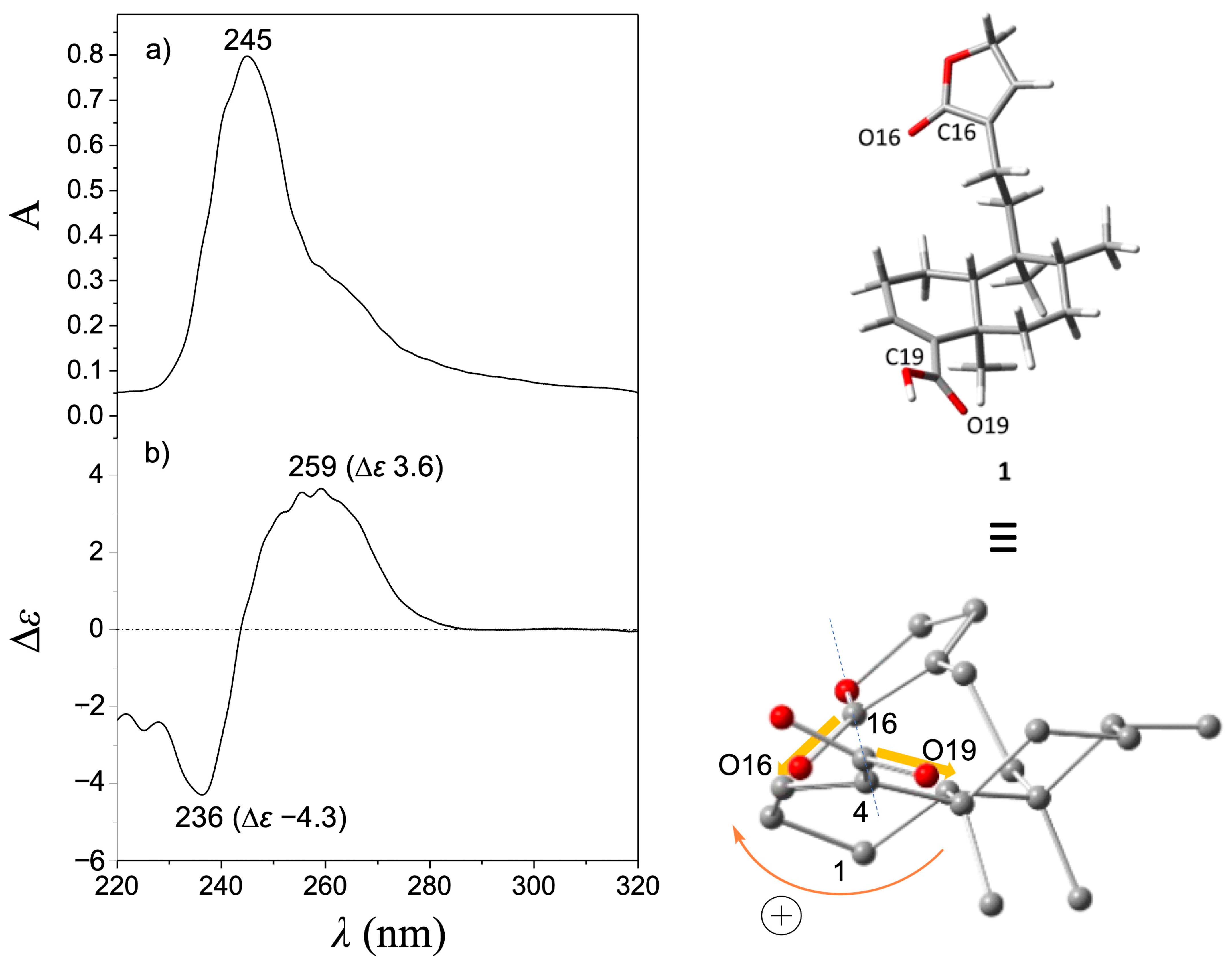
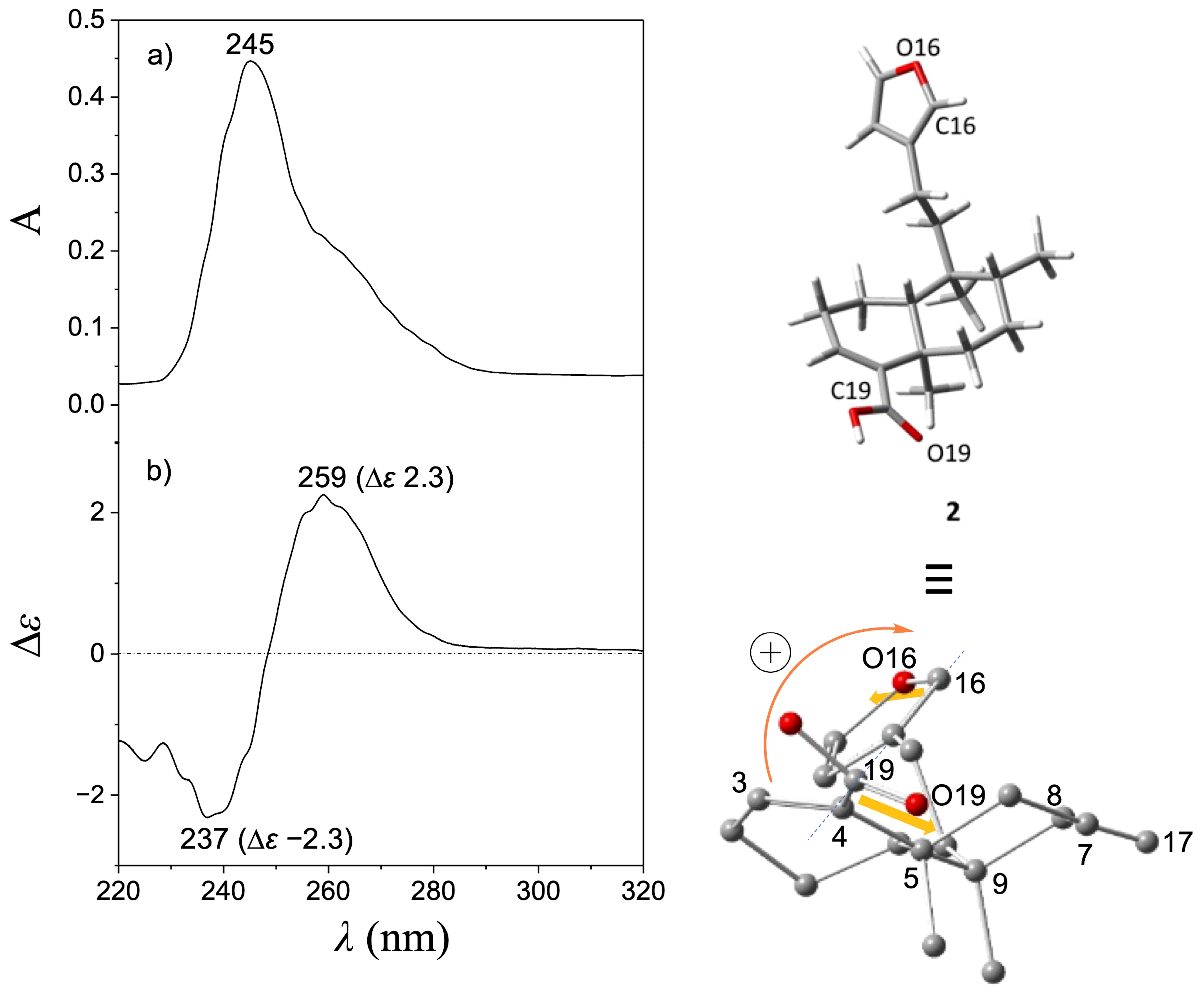
Treatment of (−)-2 with MCPBA was conducted to promote oxidation of the furan ring to yield the lactone moiety, thereby obtaining (−)-1 in a single step. This chemical strategy has been successfully employed in other molecules to obtain lactone moieties from furans [26,27,28]. The post-reaction mixture (Figure S7) was purified by column chromatography using hexanes-EtOAc mixtures (9:1, 4:1, 7:3, and 3:2) as the eluent, and silica gel (6 g) as the stationary phase to give pure (−)-1 in 18% yield. The EIMS showed a quasimolecular ion at m/z 314, corresponding to the formula C20H26O3 for [M-H2O]+ (Figure S5). The IR spectrum showed vibrational bands at 1749 and 1674 cm−1, attributed to a couple of C=O moieties (Figure S6). The 1H NMR (Figures S1 and S2) revealed a triplet at δ 7.11 (J = 1.5 Hz, H-14) and a doublet at δ 4.78 (J = 1.5 Hz, CH2-15), indicating the oxidation of the furan moiety. The COSY experiment (Figure S3) revealed a correlation between H-14 and H-15, thus confirming the chemical transformation of the furan moiety. The 13C NMR spectrum (Figure S4) revealed a carbonyl signal at δ 174.6 (C16); the vinylic carbons appeared at δ 143.7 and 135.0 (C14 and C13, respectively), and a methylene signal was observed at δ 70.3 (C15), evidencing the formation of an α,β-unsaturated lactone moiety. These spectroscopic data were concordant with those reported for patagonic acid (1), either with dextrogyre [18] or levogyre [5,13,14,17] specific rotation value. Additionally, the 13C signals at δ 37.7 (C5), 36.4 (C8), 38.9 (C9), and 46.8 (C10) observed for 1 were similar when compared with data from 2; thus, the same relative configuration was ensured [29,30]. In addition, lactone 1 displayed a specific rotation of [α]D−72 (c 0.7, CHCl3), in accordance with data from (−)-1 isolated from Baccharis patagonica, Callicarpa hypoleucophylla, Callicarpa integerrima, Casearia sylvestris, Copaifera cearensis, Croton megistocarpus, Dodonea viscosa, Echinodorus macrophyllus, Eperua leucantha, Grangea maderaspatana, Nanuza plicata, and Otostegia persica. Consequently, the same AC of (−)-1 from those vegetal species is implied. The electronic circular dichroism (ECD) analysis of (−)-patagonic acid (1) was achieved to determine its AC. This chiroptical tool has been successfully used for the AC determination of clerodane-type diterpenoids, including those with furan or lactone moieties [31,32,33,34]. The ECD spectrum of (−)-1 revealed an exciton chirality (EC) phenomenon, where a positive bisignate ECD band is observed at λmax 259 nm (∆ε 3.6) and the negative bisignate ECD band is observed at λmax 236 nm (∆ε −4.3), attributed to the interactions between the α,β-unsaturated lactone and the α,β-unsaturated carboxylic acid [35,36] (Figure 2). The ECD of (−)-2 showed a similar band absorption pattern (Figure 3) since the first Cotton effect is observed at λmax 259 nm (∆ε 2.3) and the second Cotton effect is observed at λmax 237 nm (∆ε −2.3), attributed to the interactions between the α,β-unsaturated carboxylic acid and furan chromophores [37].
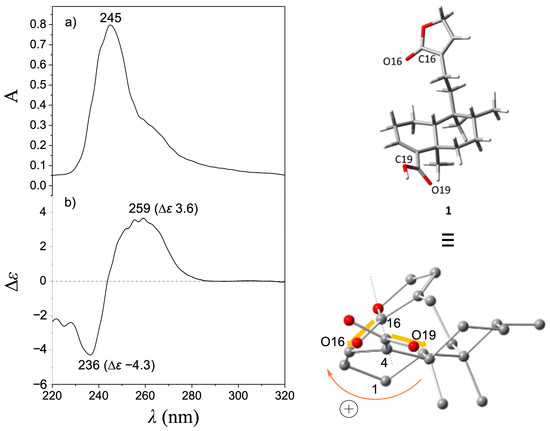
Figure 2.
(a) UV and (b) ECD spectra of (−)-patagonic acid (1). The global minimum energy conformer of (−)-1, calculated at the DFT B3LYP/DGDZVP level of theory, is shown in the upper right corner, while in the lower right corner, this conformer was rotated to visualize the positive O19-C19---C16-O16 dihedral angle (165.5°).
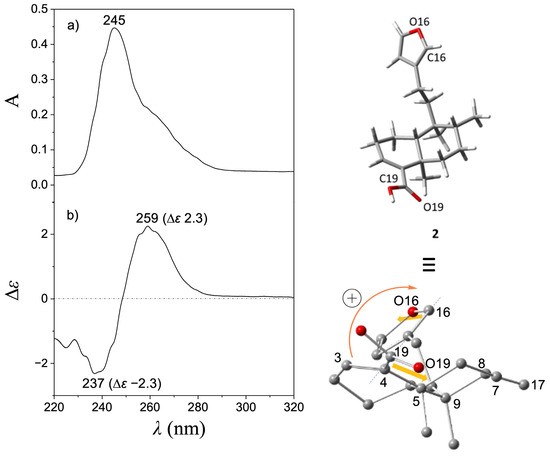
Figure 3.
(a) UV and (b) ECD spectra of (−)-hardwickiic acid (2). The global minimum energy conformer of (−)-2, calculated at the DFT B3LYP/DGDZVP level of theory, is shown in the upper right corner, while in the lower right corner, this conformer was rotated to visualize the positive O19-C19---C16-O16 dihedral angle (145.5°).
Computational calculations to approach the conformational preferences for (−)-1 and (−)-2 to be associated with the observed EC phenomenon were performed. For this purpose, molecular models for (−)-1 and (−)-2 were independently constructed, and a conformer distribution analysis was implemented using the Monte Carlo protocol at Merck Molecular Force Field (MMFF) level. Subsequently, the energies of conformers in the 0–5 kcal/mol gap were optimized at the DFT B3LYP/6-31G(d) level of theory. It follows that those conformers in the 0–3 kcal/mol energy window were geometry-optimized at the B3LYP/DGDZVP level of theory (Figures S11 and S12). This methodology has been successfully used for the conformational analysis of diterpene-type compounds [24,38,39]. Finally, the O19-C19---C16-O16 dihedral angle of the global minimum conformers of (−)-1 and (−)-2 was measured and associated with the EC results (Figure 2 and Figure 3). According to the population percentage, the Boltzmann-average dihedral angle value was also estimated using the ∆G = −RT ln K equation. Herein, the O19-C19---C16-O16 dihedral angle value for the global minimum conformer from (−)-1 was 165.5° (Table S1), while the Boltzmann-average value was 79.9°, consistent with a positive EC phenomenon. In its part, the O19-C19---C16-O16 dihedral angle from the global minimum conformer from (−)-2 was 145.5° (Table S2) and the Boltzmann-average value was 54.9°. These results suggested similar conformational preferences, which is expected due to structural differences between (−)-1 and (−)-2 being limited in the level of oxidation at the furanoid ring. Due to the experimental NMR data for (−)-1 being in full agreement with that described for (+)-1 ([α]D + 109 (c = 2.0, CHCl3)) from Sindora sumatrana and Duranta repens [18], the AC of the dextrogyre enantiomer can be established as (5S,8S,9R,10S)-(+)-patagonic acid. In addition, the calculated Boltzmann weighting for compounds (−)-1 and (−)-2 could also contribute to their potential pharmacological applications, where a direct temperature-dependence for an enzyme–substrate interaction could be considered as the literature suggested [40]; however, conformational temperature dependence of proteins should also be considered [41], but these topics could be addressed in future research.
Many natural products are chiral small molecules that can interact in different ways with biological targets. It is considered that most plants synthesize enantiomerically pure natural products; however, there are reports indicating the isolation of chiral mixtures [42,43,44], or the presence of enantiomers from different plant families [45]. It is also documented that there are differences in the biological properties of natural enantiomers [46,47,48]. Thus, it could be suggested that differences in the bioactivities of (−)-1 and (+)-1 are related to its absolute configuration.
3. Conclusions
The absolute configuration of (−)-patagonic acid (1) was successfully determined by chemical correlation with (−)-hardwickiic acid as the key starting material, as well as by an ECD experimental protocol, resulting in the 5R,8R,9S,10R configuration for (−)-1. Since the NMR data of (−)-1 are identical to those of (+)-1, the absolute configuration of dextrogyre patagonic acid can be inferred as 5S,8S,9R,10S. Consequently, this research complements the literature on the configurational studies of clerodane diterpene-type compounds.
4. Materials and Methods
4.1. General Experimental Procedures
The reagent MCPBA (273031) was acquired from Sigma-Aldrich® (Toluca, Mexico), and HCl 36.5–38.0% (9535) from J.T. Baker, and used as purchased. Solvents were distilled prior to use. IR spectra were obtained on a Nicolet 6700 FTIR spectrometer by Thermo Scientific (Waltham, MA, USA), using the attenuated total reflectance method and are reported in wavenumbers (cm−1). Optical rotations were determined on a Perkin Elmer 341 polarimeter (Norwalk, Connecticut, E.U.A) using CHCl3 solutions at 25 °C. ECD spectra were obtained on a stand-alone JASCO CD-2095 circular dichroism detector (Cremella, Italy) from CHCl3 solutions of (−)-1 or (−)-2 (250 µg/mL each). NMR spectra were measured at 400 MHz for 1H and 100 MHz for 13C on a Varian Mercury Plus 400 spectrometer (Varian Inc., Palo Alto, CA, USA). Also, 1H spectra were measured on a Varian Mercury 300 spectrometer (Varian Inc., Palo Alto, CA, USA). In both cases, CDCl3 solutions at 298 K were prepared, using tetramethylsilane as the internal reference or residual CHCl3 (δ = 7.26 for 1H or 77.16 for 13C). Chemical shift values (δ) are reported in parts per million and coupling constants (J) are given in Hz. Column chromatography was carried out on Merck Silica 60 (230–400 mesh).
4.2. Plant Material
Specimens of Chromolaena pulchella were collected near km 2 of Tiripetío–Carácuaro–Eréndira state road (MICH-25) in the municipality of Madero, State of Michoacán, Mexico (N 19.5020299, W−101.348405). A specimen was deposited (IEB 192522) at the Herbarium of the Instituto de Ecología, A.C., Centro Regional del Bajío, Pátzcuaro, Michoacán, Mexico.
4.3. Extraction and Isolation of (−)-Hardwickiic Acid (2)
(−)-Hardwickiic acid (2) was isolated from C. pulchella as previously described [18]. Briefly, air-dried flowers (1.2 kg) were extracted with hexanes (8 L) under reflux for 6 h. The solvent was removed under reduced pressure, and the crude extract (60 g) was obtained as a yellow oil. A portion of the crude extract (2.5 g) was subjected to column chromatography (2 cm diameter) using silica gel as the stationary phase (16 g) and gradients of hexanes-acetone (100:0, 99:1, 49:1, and 24:1) as the eluent. Fractions of 5 mL each were recovered. Fractions 80–100 from polarity 49:1 (228 mg) were subjected to column chromatography using silica gel-AgNO3 (6:1) as the stationary phase and eluted with hexanes-AcOEt (46:4) (fractions of 10 mL each). Compound 2 was obtained as a colorless oil in fractions 6–13 (57 mg). The physical and spectroscopic data agree with those described [24,25].
4.4. Chemical Correlation of (−)-Patagonic Acid (1) from (−)-Hardwickiic Acid (2)
A solution of (−)-2 (50 mg, 0.16 mmol, 0.5 eq) in CHCl3 (2 mL) was prepared and cooled at 0 °C. Afterwards, HCl 36.5-38.0% (0.1 mL) followed by MCPBA (39 mg, 0.23 mmol, 1.1 eq) were added, and the mixture was stirred at 0 °C for 2 h. The solvent was removed, and the crude reaction was dissolved in EtOAc (20 mL). The organic layer was washed with saturated aqueous NaHCO3 solution (3 × 20 mL) and H2O (3 × 20 mL), dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The resulting mixture was purified by column chromatography using silica gel as stationary phase (6 g in a 1.5 cm diameter column) and gradients of hexanes-EtOAc (9:1, 4:1, 7:3, 3:2), in volume, as eluent, obtaining volume fractions of 4 mL each. (−)-Patagonic acid (1) was obtained as colorless oil (10 mg, 18%), in fractions 100-160 eluted with the 7:3 and 3:2 mixtures. [α]D −72 (c 0.7, CHCl3), lit. [α]D −65.9 (c 2.65, CHCl3) [5]. EIMS (70 eV) m/z (rel. int.) 314 [M-H2O]+ (100), 175 (61), 139 (80), 125 (65), 107 (44), 105 (69), 93 (53); lit. 314 (64), 175 (19), 125 (27), 105 (27), 93 (26) [5]. 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) are concordant with the data described [5,13,14,17].
4.5. Conformational Analysis
Molecular models of (−)-1 and (−)-2 were independently constructed in silico using the Spartan ’04 program and subjected to the Monte Carlo protocol using the Merck Molecular Force Field (MMFF94) as implemented in the Spartan’04 program. A total of 65 and 61 conformers resulted for (−)-1 and (−)-2, respectively, in an energy window of 0–10 kcal/mol. Conformers in the 0–5 kcal/mol energy gap [13 conformers for (−)-1 and 15 conformers for (−)-2] were selected for each compound and submitted for single-point energy calculations at the B3LYP/6-31G(d) level of theory in the Spartan’04 program. Conformers in the 0-3 kcal/mol gap [6 conformers for (−)-1 and 7 conformers for (−)-2] were geometry-optimized at the DFT B3LYP/DGDZVP level of theory in the Gaussian 09 program and visualized in the GaussView 6.0 graphical interface. The O19-C19---C16-O16 dihedral angle was measured for each conformer and weight averaged according to the ∆G = −RT ln K equation. Conformers in the 0–3 kcal/mol and thermochemical analysis for compounds (−)-1 and (−)-2 can be found in the Supplementary Materials (Figures S9 and S10, and Tables S1 and S2). Geometry optimization required some 10 h of CPU time per conformer when using a CPU ES-2680 v3 with 110 Gb RAM operating at 2.5 GHz × 48 core processors.
Supplementary Materials
NMR, IR, EIMS spectra and thermochemical analysis for (−)-1 and (−)-2. Figure S1. 1H NMR spectrum of (−)-patagonic acid (1) (300 MHz, CDCl3, 40 scans). Figure S2. Expansion of 1H NMR spectrum of (−)-patagonic acid (1) (300 MHz, CDCl3). Figure S3. H-H COSY spectrum of (−)-patagonic acid (1) (400 MHz, CDCl3, 8 scans). Figure S4. 13C NMR spectrum of (−)-patagonic acid (1) (100 MHz, CDCl3, 1984 scans). Figure S5. EIMS spectrum of (−)-patagonic acid (1). Figure S6. IR spectrum of (−)-patagonic acid (1). Figure S7. 1H NMR spectrum of the reaction mixture of (−)-hardwickiic (2) acid with MCPBA (400 MHz, CDCl3, 24 scans). Figure S8. 1H NMR spectrum of (−)-hardwickiic acid (2) (400 MHz, CDCl3, 40 scans). Figure S9. Expansion of 1H NMR spectrum of (−)-hardwickiic acid (2) (400 MHz, CDCl3). Figure S10. 13C NMR spectrum of (−)-hardwickiic acid (2) (100 MHz, CDCl3, 1984 scans). Figure S11. Calculated conformers of (−)-patagonic acid (1) within the 0–3 kcal/mol energy gap with B3LYP/DGDZVP basis set. Gibbs free energies relative to 1a for B3LYP/GDGDZVP = −677,467.100 kcal/mol. Figure S12. Calculated conformers of (−)-hardwickiic acid (2) within the 0–3 kcal/mol energy gap with B3LYP/DGDZVP basis set. Gibbs free energies relative to 1a for B3LYP/GDGDZVP = −630,238.950 kcal/mol. Table S1. Thermochemical analysis of (−)-patagonic acid (1) and dihedral angle values for O19-C19---C16-O16. Table S2. Thermochemical analysis of (−)-hardwickiic acid (2) and dihedral angle values for O19-C19---C16-O16.
Author Contributions
Conceptualization, A.T.-A. and M.A.G.-H.; Methodology E.E.S.-G., H.J.P.-I. and A.J.O.-O.; Validation, G.R.-G., Y.L. and B.Y.B.-G.; Formal Analysis, C.M.C.-G.-R. and C.T.; Writing—Original Draft Preparation, A.T.-A.; Writing—Review and Editing, M.A.G.-H., R.E.d.R. and C.M.C.-G.-R.; Data Curation, G.R.-G. and Y.L.; Supervision, R.E.d.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by CIC-UMSNH and FCCHT123_ME-4.1-0008.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
E.E.S.-G., A.J.O.-O., and H.J.P.-I. are grateful to SECIHTI-Mexico for scholarships 764722, 800679, and 1268656, respectively. We are grateful to Q. F. B. Verónica Reyes Olivares for optical rotation measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lima, J.R.; Marinho, E.M.; Alencar de Menezes, J.E.S.; Mendes, F.R.S.; da Silva, A.W.; Ferreira, M.K.A.; Santos, O.L.; Barbosa, I.M.; Marinho, E.S.; Marinho, M.M.; et al. Biological properties of clerodane-type diterpenes. J. Anal. Pharm. Res. 2022, 11, 56–64. [Google Scholar]
- Martínez-Casares, R.M.; Hernández-Vázquez, L.; Mandujano, A.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Pérez-Ramos, J. Anti-inflammatory and cytotoxic activities of clerodane-type diterpenes. Molecules 2023, 28, 4744. [Google Scholar] [CrossRef]
- Hagiwara, H. Total syntheses of clerodane diterpenoids—A review. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef]
- Li, R.; Morris-Natschkeb, S.L.; Lee, K.-H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Rivera, A.P.; Fiani, F.; Castillo, M. 15α-Hydroxy-β-amyrin and patagonic acid from Baccharis magellanica and Baccharis patagonica. J. Nat. Prod. 1988, 51, 155–157. [Google Scholar] [CrossRef]
- Kobayashi, J.; Sekiguchi, M.; Shigemori, H.; Ohsaki, A. Echinophyllins A and B, novel nitrogen-containing clerodane diterpenoids from Echinodorus macrophyllus. Tetrahedron Lett. 2000, 41, 2939–2943. [Google Scholar] [CrossRef]
- Singh, P.; Jain, S.; Jakupovic, J. Clerodane derivatives from Grangea maderaspatana. Phytochemistry 1988, 27, 1537–1539. [Google Scholar] [CrossRef]
- Mora, S.; Castro, V.; Poveda, L.; Chavarría, M.; Murillo, R. Two new 3,4-seco-ent-kaurenes and other constituents from the Costa Rican endemic species Croton megistocarpus. Helv. Chim. Acta 2011, 94, 1888–1892. [Google Scholar] [CrossRef]
- Pinto, A.C.; Braga, W.F.; Rezende, C.M.; Garrido, F.M.S.; Veiga, V.F.; Bergter, L.; Patitucci, M.L.; Antunes, O.A.C. Separation of acid diterpenes of Copaifera cearensis Huber ex Ducke by flash chromatography using potassium hydroxide impregnated silica gel. J. Braz. Chem. Soc. 2000, 11, 355–360. [Google Scholar] [CrossRef]
- Avila, D.; Medina, J.D.; Deeming, A.J. A new clerodane-type diterpenoid from Eperua leucantha. J. Nat. Prod. 1992, 55, 845–850. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, J.-J.; Chen, S.-R.; Hwang, T.-L.; Fang, S.-Y.; Korinek, M.; Chen, C.-Y.; Lin, Y.-S.; Wu, T.-Y.; Yen, M.-H.; et al. Clerodane diterpenoids from Callicarpa hypoleucophylla and their anti-inflammatory activity. Molecules 2020, 25, 2288. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.-W.; Zhao, Y.-X.; Qiu, X.; Zhang, X.-C.; Zhou, Y.-L.; Zeb, M.A.; Pang, W.-H.; Li, R.; Wang, M.-R.; Cheng, B.; et al. Callicarpanes A–L, twelve new clerodane diterpenoids with NLRP3 inflammasome inhibitory activity from Callicarpa integerrima. Chem. Biodivers. 2023, 20, e202200985. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.U.; Farooq, U.; Abbaskhan, A.; Hussain, J.; Abbasi, M.A.; Nawaz, S.A.; Choudhary, M.I. Four new diterpenoids from Ballota limbata. Helv. Chim. Acta 2004, 87, 682–689. [Google Scholar] [CrossRef]
- Ayatollahi, S.A.; Kobarfard, F.; Asgarpanah, J.; Rahmati, R.M.; Fanai, G.; Iqbal, C.M. Diterpenoids of Otostegia persica (Burm.) Boiss. DARU 2009, 17, 290–293. [Google Scholar]
- Wang, W.; Ali, Z.; Li, X.-C.; Smillie, T.A.; Guo, D.-A.; Khan, I.A. New clerodane diterpenoids from Casearia sylvestris. Fitoterapia 2009, 80, 404–407. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, M.-Y.; Zhou, Z.-Y.; Xu, D. Two new clerodane diterpenes from Dodonaea viscosa. Z. Naturforsch. B 2010, 65, 83–86. [Google Scholar] [CrossRef]
- Pinto, M.E.F.; da Silva, M.S.; Schindler, E.; Barbosa, F.J.M.; dos Santos, E.-B.R.; Castello-Branco, M.V.S.; de Fatima, A.M.; Fechine, T.J. 3′,8″-biisokaempferide, a cytotoxic biflavonoid and other chemical constituents of Nanuza plicata (Velloziaceae). J. Braz. Chem. Soc. 2010, 21, 1819–1824. [Google Scholar] [CrossRef]
- Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyadna, S. Constituents of Sindora sumatrana MIQ. III. New trans-clerodane diterpenoids from the dried pods. Chem. Pharm. Bull. 1994, 42, 1202–1207. [Google Scholar] [CrossRef]
- Jung, H.J.; Chung, S.Y.; Nam, J.W.; Chae, S.W.; Lee, Y.-J.; Seo, E.-K.; Lee, H.J. Inhibition of P-glycoprotein-induced multidrug resistance by a clerodane-type diterpenoid from Sindora sumatrana. Chem. Biodivers. 2010, 7, 2095–2101. [Google Scholar] [CrossRef]
- Jang, D.S.; Min, H.-Y.; Jeong, Y.-H.; Lee, S.K.; Seo, E.-K. Di-and sesqui-terpenoids isolated from the pods of Sindora sumatrana and their potential to inhibit lipopolysaccharide-induced nitric oxide production. Arch. Pharm. Res 2004, 27, 291–294. [Google Scholar] [CrossRef]
- Iqbal, K.; Malik, A.; Mukhtar, N.; Anis, I.; Khan, S.N.; Choudhary, M.I. α-Glucosidase inhibitory constituents from Duranta repens. Chem. Pharm. Bull. 2004, 52, 785–789. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batista, J.M.; Da Silva Bolzani, V. Determination of the absolute configuration of natural product molecules using vibrational circular dichroism. Stud. Nat. Prod. Chem. 2014, 41, 383–417. [Google Scholar] [CrossRef]
- Kong, L.Y.; Wang, P. Determination of the Absolute Configuration of Natural Products. Chin. J. Nat. Med. 2013, 11, 193–198. [Google Scholar] [CrossRef]
- Gómez-Hurtado, M.A.; Torres-Valencia, J.M.; Manríquez-Torres, J.; del Río, R.E.; Motilva, V.; García-Mauriño, S.; Ávila, J.; Talero, E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P. Absolute configuration of labdanes and ent-clerodanes from Chromolaena pulchella by vibrational circular dichroism. Phytochemistry 2011, 72, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Pandey, R.C.; Dev, S. The absolute stereochemistry of hardwickiic acid and its congeners. Tetrahedron Lett. 1968, 9, 2681–2684. [Google Scholar] [CrossRef]
- Huang, B.; Guo, L.; Jia, Y. Protecting-group-free enantioselective synthesis of (−)-pallavicinin and (+)-neopallavicinin. Angew. Chem. Int. Ed. 2015, 54, 13599–13603. [Google Scholar] [CrossRef]
- Badovskaya, L.A.; Povarova, L.V. Oxidation of furans (Review). Chem. Heterocycl. Compd. 2009, 45, 1023–1034. [Google Scholar] [CrossRef]
- Talavera-Alemán, A.; Gómez-Hurtado, M.A.; Rodríguez-García, G.; Ochoa-Zarzosa, A.; Thomassigny, C.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; del Río, R.E. Preparation and cytotoxic evaluation of vouacapane oxidation products. Heterocycles 2020, 100, 207–224. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Structural revision of tinotufolins from Tinospora crispa leaves guided by empirical rules and DFT calculations. J. Nat. Prod. 2024, 87, 774–782. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. New clerodane diterpenoids from Solidago altissima and stereochemical elucidation via 13C NMR chemical shift analysis. Tetrahedron 2022, 110, 132691. [Google Scholar] [CrossRef]
- Bellier, T.G.; Fomo, F.F.A.; Mas-Claret, E.; Langat, M.K.; Frese, M.; Nouga, B.A.; Duplex, W.A.; Kamdem, W.A.F.; Sewald, N.; Ndjakou, L.B. Cytotoxic clerodane diterpenoids from the roots of Casearia barteri Mast. RSC Adv. 2024, 14, 23109–23117. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Andrade-Cetto, A.; Rosas-Ramírez, D.G.; Arreguín-Espinosa, R. Phytochemical screening and isolation of new ent-clerodane diterpenoids from Croton guatemalensis Lotsy. Plants 2022, 11, 3159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, Z.; Chen, C.; Wang, H.; Liu, H.; Li, J.; Sun, C.; Lou, H.; Pan, W. New clerodane diterpenoids from Callicarpa pseudorubella and their antitumor proliferative activity. Fitoterapia 2024, 174, 105878. [Google Scholar] [CrossRef]
- You, J.-Q.; Liu, Y.-N.; Zhou, J.-S.; Sun, X.-Y.; Lei, C.; Mu, Q.; Li, J.-Y.; Hou, A.-J. cis-Clerodane Diterpenoids with structural diversity and anti-inflammatory activity from Tinospora crispa. Chin. J. Chem. 2022, 40, 2882–2892. [Google Scholar] [CrossRef]
- Weiss, U.; Ziffer, H. Cotton effects of α,β-unsaturated carboxylic acids. J. Org. Chem. 1963, 28, 1248–1251. [Google Scholar] [CrossRef]
- Beecham, A.F. The CD of αβ-unsaturated lactones. Tetrahedron 1972, 28, 5543–5554. [Google Scholar] [CrossRef]
- Nyulászi, L. Near UV spectra of furan and its derivatives. J. Mol. Struct. 1992, 273, 133–138. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Montaño-Hernández, P.Y.; Salas-Huerta, O.; Ramírez-González, D.I.; Pérez-Juanchi, D.; Torres-Medicis, J.P.; Macías-Rubalcava, M.L.; Bedolla-García, B.Y.; Zamudio, S.; Quijano, L.; et al. Phytotoxic neo-clerodane and rearranged neo-clerodane type diterpenoids from Salvia albiflora. Tetrahedron 2025, 174, 134490. [Google Scholar] [CrossRef]
- Rasyid, F.A.; Fukuyoshi, S.; Ando, H.; Miyake, K.; Atsumi, T.; Fujie, T.; Saito, Y.; Goto, M.; Shinya, T.; Mikage, M.; et al. A novel clerodane diterpene from Vitex cofassus. Chem. Pharm. Bull. 2017, 65, 116–120. [Google Scholar] [CrossRef]
- Katiyar, A.; Thompson, W.H. Temperature dependence of peptide conformational equilibria from simulations at a single temperature. J. Phys. Chem. A 2021, 125, 2374–2384. [Google Scholar] [CrossRef]
- Dong, M. A Minireview on Temperature dependent protein conformational sampling. Protein J. 2021, 40, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Rositano, V.; Senaldi, L.; Bernardi, A.; Allegrini, P.; Appendino, G. Scalemic natural products. Nat. Prod. Rep. 2023, 40, 1647–1671. [Google Scholar] [CrossRef] [PubMed]
- Arreaga-González, H.M.; Oliveros-Ortiz, H.J.; del Río, R.E.; Rodríguez-García, G.; Torres-Valencia, J.M.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Methodology for the absolute configuration determination of epoxythymols using the constituents of Piptothrix areolare. J. Nat. Prod. 2021, 84, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Arreaga-González, H.M.; Pardo-Novoa, J.C.; del Río, R.E.; Rodríguez-García, G.; Torres-Valencia, J.M.; Manríquez-Torres, J.J.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Methodology for the absolute configuration determination of epoxythymols using the constituents of Ageratina glabrata. J. Nat. Prod. 2018, 81, 63–71. [Google Scholar] [CrossRef]
- Finefield, J.M.; Sherman, D.H.; Kreitman, M.; Williams, R.M. Enantiomeric Natural Products: Occurrence and Biogenesis. Angew. Chem. Int. Ed. 2012, 51, 4802–4836. [Google Scholar] [CrossRef]
- Yu, J.-H.; Zhai, H.-J.; Yu, Z.-P.; Zhang, Q.-Q.; Ge, Y.-X.; Zhang, Y.-Y.; Jiang, C.-S.; Zhang, H. Methyl 2-naphthoates from a traditional Chinese herb Morinda officinalis var. officinalis. Tetrahedron 2009, 75, 3793–3801. [Google Scholar] [CrossRef]
- Yu, J.-H.; Yu, Z.-P.; Capon, R.J.; Zhang, H. Natural Enantiomers: Occurrence, Biogenesis and Biological Properties. Molecules 2022, 27, 1279. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Franchini, A.; De Luca, M.; Saturnino, C.; Andreu, I.; Sinicropi, M.S.; Catalano, A. A Look at the Importance of Chirality in Drug Activity: Some Significative Examples. Appl. Sci. 2022, 12, 10909. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).