N-(2-((2-(1H-indol-3-yl)ethyl)carbamoyl)phenyl)furan-2-carboxamide
Abstract
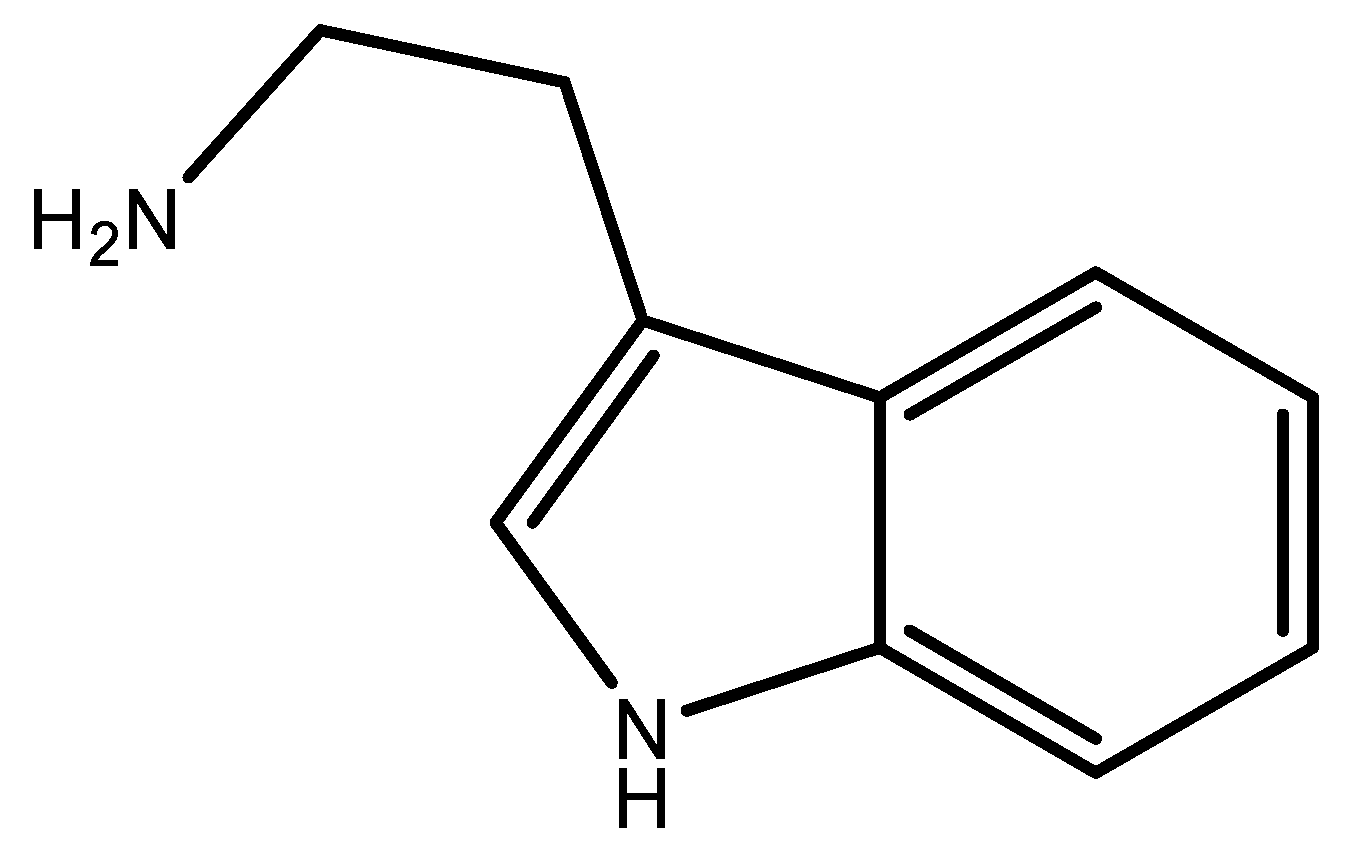
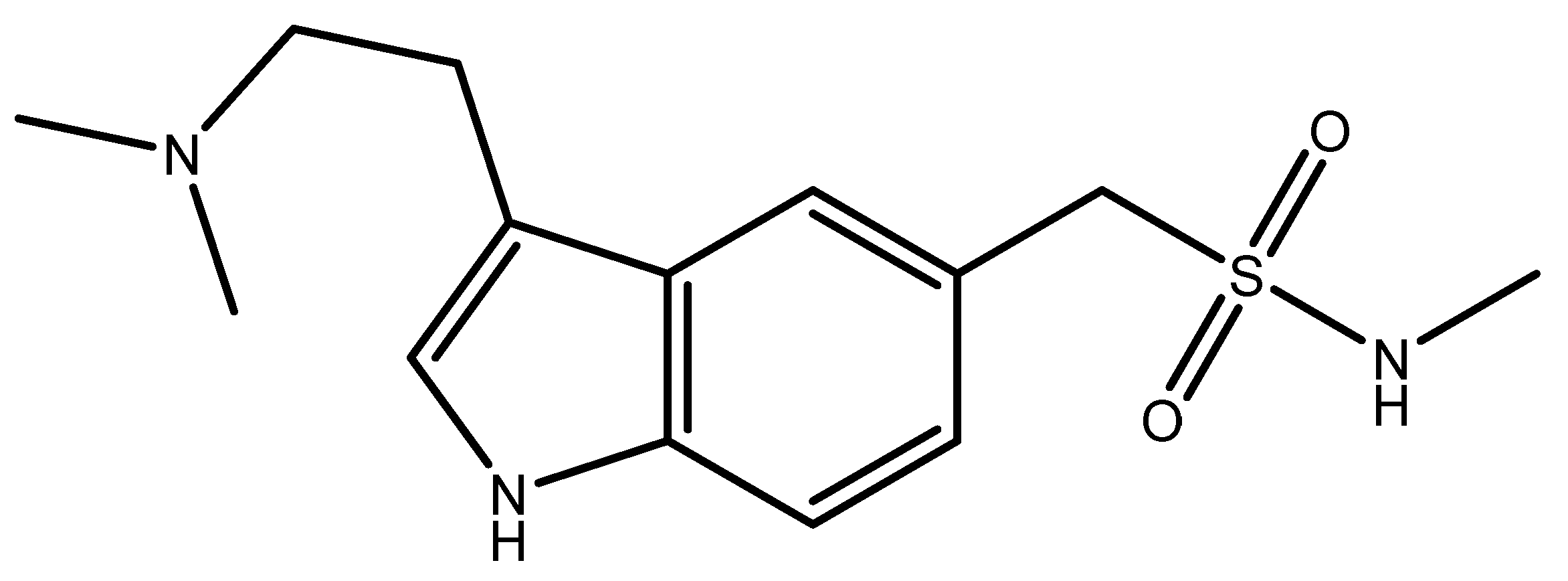
1. Introduction
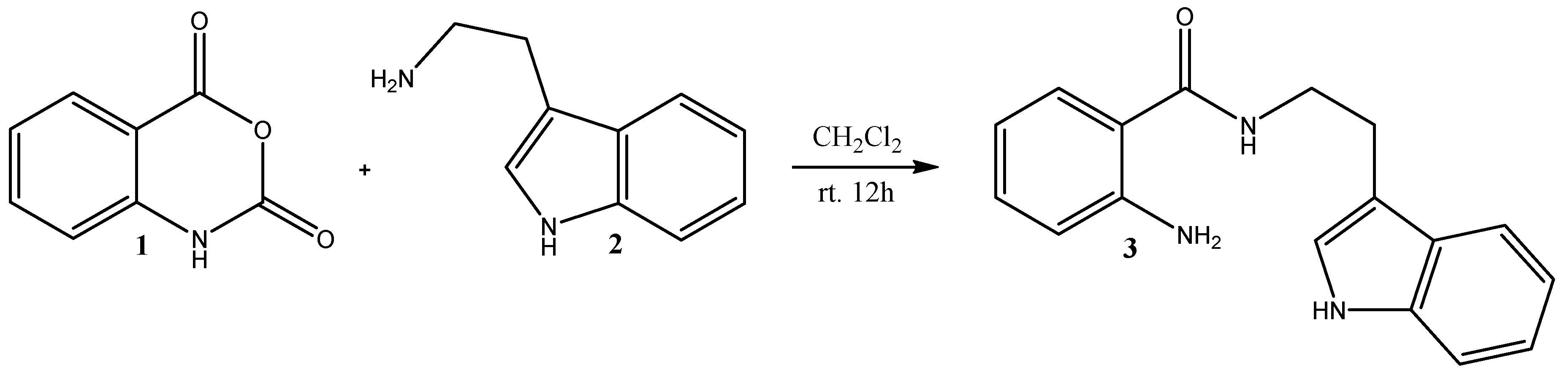
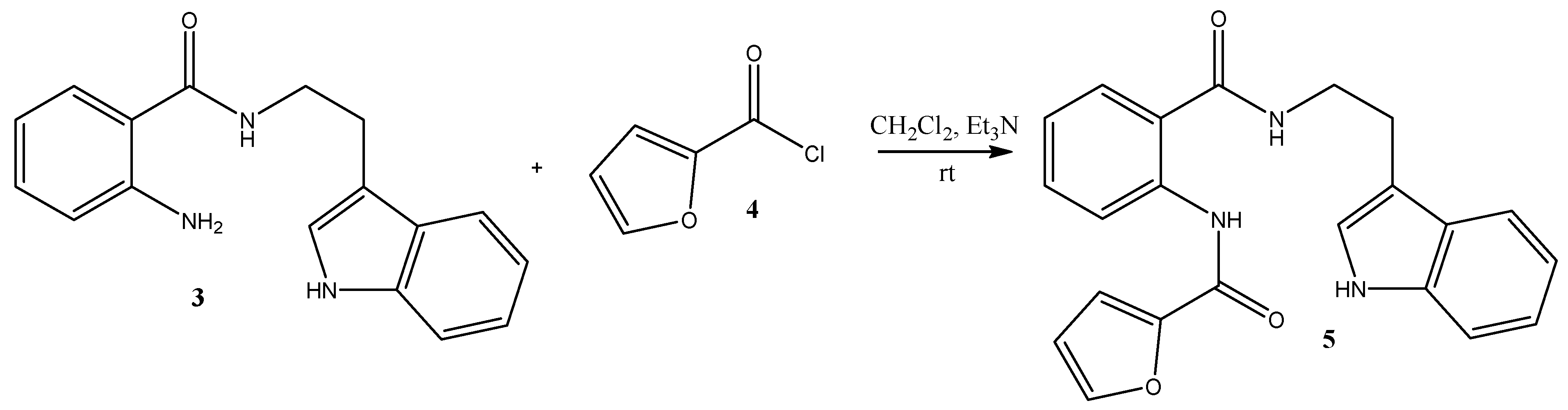
2. Results and Discussion
3. Materials and Methods
Synthesis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jirjees, V.; Al–Hamdani, A. Synthesis, characterization and evaluation of anticancer and antioxidant activity of new azo dye derivatives from tryptamine and complexes. Bull. Chem. Soc. Ethiop. 2025, 7, 1317–1334. [Google Scholar] [CrossRef]
- Bektas, H.; Demirbas, A.; Demirbas, N.; Karooglu, S.A. Synthesis and biological activity studies of new hybrid molecules containing tryptamine moiety. Med. Chem. Res. 2012, 21, 212–223. [Google Scholar] [CrossRef]
- Hemachandran, K.; Anbusrinivasan, P.; Ramalingam, S.; Manoharan, C.; Aarthi, R. Biological and structural properties’ interpretation on antitumour drug 3-(2-aminoethyl) indole (tryptamine) using molecular spectroscopy and computational tools. J. Taibah Univ. Med. Sci. 2018, 1, 231–247. [Google Scholar] [CrossRef]
- Lancianesi, S.; Palmieri, A.; Petrini, M. Synthetic Approaches to 3-(2-Nitroalkyl) Indoles and Their Use to Access Tryptamines and Related Bioactive Compounds. Chem. Rev. 2014, 14, 7108–7149. [Google Scholar] [CrossRef]
- Simonetti, G.; Boga, C.; Durante, J.; Micheletti, G.; Telese, D.; Caruana, P.; Ghelli Luserna di Rorà, A.; Mantellini, F.; Bruno, S.; Martinelli, G.; et al. Synthesis of Novel Tryptamine Derivatives and Their Biological Activity as Antitumor Agents. Molecules 2021, 26, 683. [Google Scholar] [CrossRef]
- Asghar, S.; Mushtaq, N.; Ahmad, A.; Munawwar, R.; Ansari, S.; Rizvi, S. Design, synthesis and therapeutic investigation of tryptamine derivatives as potential antioxidant and amyloid inhibitor/disaggregator. Res. J. Pharm. Technol. 2023, 8, 3622–3632. [Google Scholar] [CrossRef]
- Singh, T.; Singh, O. Recent Progress in Biological Activities of Indole and Indole Alkaloids. Med. Chem. 2018, 1, 9–25. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, Y.; Peng, Y.; Rashid, H.; Quan, W.; Xie, P.; Wu, L.; Jiang, J.; Wang, L.; Liu, X. Design, synthesis and evaluation of novel (S)-tryptamine derivatives containing an allyl group and an aryl sulfonamide unit as anticancer agents. Bioorg. Med. Chem. Lett. 2019, 9, 1133–1137. [Google Scholar] [CrossRef]
- Klochkov, S.G.; Afanas’eva, S.V.; Bulychev, Y.N.; Neganova, M.E.; Shevtsova, E.F. Synthesis and biological activity of isoalantolactone-tryptamine conjugates. Russ. Chem. Bull. 2012, 61, 409–415. [Google Scholar] [CrossRef]
- Guerrero, T.; Juárez-Cerrillo, S.; Matus, M.; Durand-Niconoff, J. Study of Local Reactivity of Isatoic Anhydride Applying Quantum Molecular Similarity. ChemistrySelect 2021, 6, 4390. [Google Scholar] [CrossRef]
- Mabkhot, Y.; Al-Majid, A.; Barakat, A.; Al-Showiman, S.; Al-Har, M.; Radi, S.; Naseer, M.; Hadda, T. Synthesis and Biological Evaluation of 2-Aminobenzamide Derivatives as Antimicrobial Agents: Opening/Closing Pharmacophore Site. Int. J. Mol. Sci. 2014, 15, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, A.; Mironov, V. Synthesis of isatoic anhydride derivatives (micro review). Chem. Heterocycl. Comp. 2016, 52, 90–92. [Google Scholar] [CrossRef]
- Tiwari, S.; Senthil, S.; Khanna, S.; Duraisamy, S.; Vechalapu, S.; Mallojjala, S.; Allimuthu, D. Tuning isatoic anhydrides’ lysine ligation chemistry for bio conjugation and drug delivery. Cell Rep. Phys. Sci. 2024, 5, 20. [Google Scholar] [CrossRef]
- Spanedda, M.; Bourel-Bonnet, L. Cyclic Anhydrides as Powerful Tools for Bioconjugation and Smart Delivery. Bioconjugate Chem. 2021, 3, 482–496. [Google Scholar] [CrossRef]
- Sayahi, M.; Adib, M.; Hamooleh, Z.; Zhu, L.; Amanlou, M. Reaction between Furan- or Thiophene-2-carbonyl Chloride, Isocyanides, and Dialkyl Acetylenedicarboxy-lates: Multicomponent Synthesis of 2,2′-Bifurans and 2-(Thiophen-2-yl)furans. Helv. Chim. Acta 2015, 98, 1231–1239. [Google Scholar] [CrossRef]
- Javed, M.; Zubair, M.; Rizwan, K.; Jamil, M. In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives. Molecules 2023, 28, 4583. [Google Scholar] [CrossRef]
- Ahmed, W.; Yan, X.; Hu, D.; Adnan, M.; Tang, R.; Cui, Z. Synthesis and fungicidal activity of novel pyrazole derivatives containing 5-Phenyl-2-Furan. Bioorg. Med. Chem. 2019, 27, 115048. [Google Scholar] [CrossRef]
- Banerjee, R.; HKS, K.; Banerjee, M. Medicinal significance of furan derivatives: A Review. Int. J. Rev. Life. Sci. 2012, 1, 7–16. [Google Scholar]
- Saeid, H.; Al-Sayed, H.; Bader, M. A Review on Biological and Medicinal Significance of Furan. AlQalam J. Med. App. Sci. 2023, 1, 44–58. [Google Scholar] [CrossRef]
- Li, X.; Lee, Y.-R. Facile One-Pot Synthesis of Quinazoline-2,4-dione Derivatives and Application to Naturally Occurring Alkaloids from Zanthoxylum arborescens. Bull. Korean Chem. Soc. 2011, 32, 2121–2124. [Google Scholar] [CrossRef]
- Li, Y.; Feng, T.; Liu, P.; Liu, C.; Wang, X.; Li, D.; Li, N.; Chen, M.; Xu, Y.; Si, S. Optimization of Rutaecarpine as ABCA1 Up-Regulator for Treating Atherosclerosis. ACS Med. Chem. Lett. 2014, 5, 884–888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrova, D.; Ivanov, I.; Manolov, S.; Bojilov, D. N-(2-((2-(1H-indol-3-yl)ethyl)carbamoyl)phenyl)furan-2-carboxamide. Molbank 2025, 2025, M2025. https://doi.org/10.3390/M2025
Dimitrova D, Ivanov I, Manolov S, Bojilov D. N-(2-((2-(1H-indol-3-yl)ethyl)carbamoyl)phenyl)furan-2-carboxamide. Molbank. 2025; 2025(3):M2025. https://doi.org/10.3390/M2025
Chicago/Turabian StyleDimitrova, Diyana, Iliyan Ivanov, Stanimir Manolov, and Dimitar Bojilov. 2025. "N-(2-((2-(1H-indol-3-yl)ethyl)carbamoyl)phenyl)furan-2-carboxamide" Molbank 2025, no. 3: M2025. https://doi.org/10.3390/M2025
APA StyleDimitrova, D., Ivanov, I., Manolov, S., & Bojilov, D. (2025). N-(2-((2-(1H-indol-3-yl)ethyl)carbamoyl)phenyl)furan-2-carboxamide. Molbank, 2025(3), M2025. https://doi.org/10.3390/M2025







