bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate
Abstract
1. Introduction
2. Results
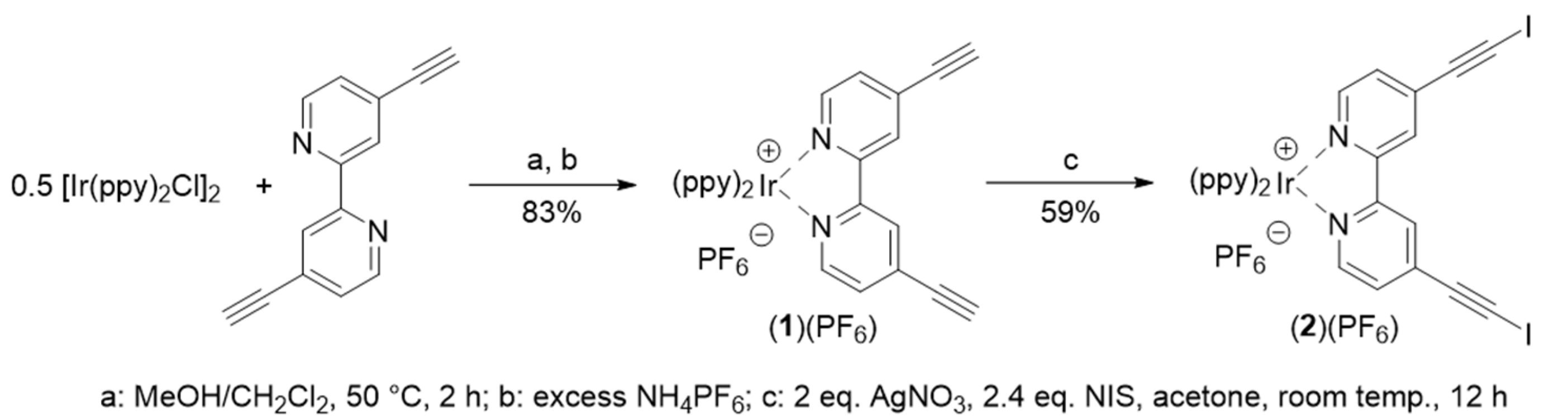
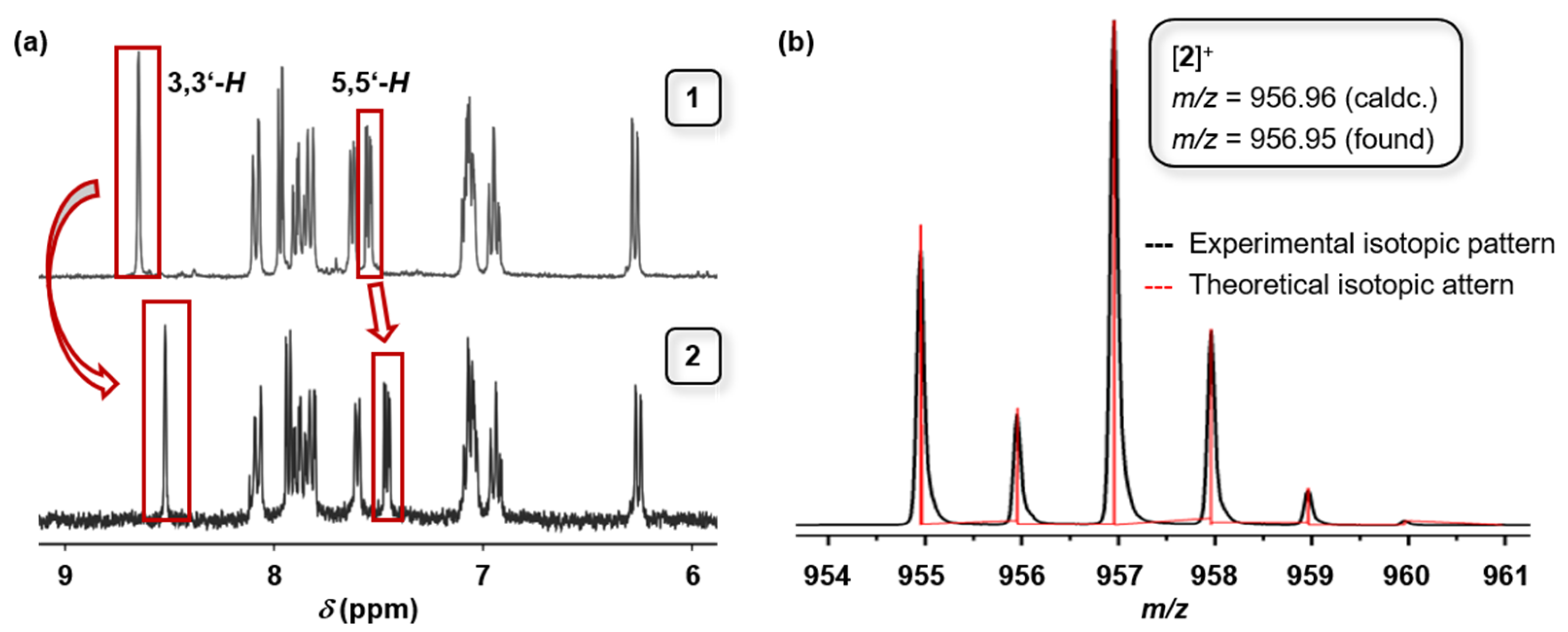
2.1. Synthesis and Characterization
2.2. Single-Crystal X-Ray Diffraction (XRD) Analysis
3. Discussion
4. Materials and Methods
4.1. General
4.2. bis(2-Phenylpyridinato-)(4,4′-diethynyl-2,2′-bipyridine)iridium(III) Hexafluorophosphate(1)(PF6)
4.3. bis(2-Phenylpyridinato-)[4,4′-bis(iodoethynyl-2,2′-bipyridine]iridium(III) Hexafluorophosphate(2)(PF6)
4.4. X-Ray Diffraction Analysis of (2)(PF6)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Colin, M. Note sur quelques combinaisons de l’iode. Ann. Chim 1814, 91, 252–272. [Google Scholar]
- Guthrie, F. On the iodide of iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef]
- Erdélyi, M.; Esterhuysen, C.; Zhu, W. Halogen bonding: From fundamentals to applications. ChemPlusChem 2021, 86, 1229–1230. [Google Scholar] [CrossRef]
- Kampes, R.; Meurer, J.; Hniopek, J.; Bernt, C.; Zechel, S.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Exploring the principles of self-healing polymers based on halogen bond interactions. Front. Soft Matter 2022, 2, 973821. [Google Scholar] [CrossRef]
- Tepper, R.; Bode, S.; Geitner, R.; Jäger, M.; Görls, H.; Vitz, J.; Dietzek, B.; Schmitt, M.; Popp, J.; Hager, M.D.; et al. Polymeric halogen-bond-based donor systems showing self-healing behavior in thin films. Angew. Chem. Int. Ed. 2017, 56, 4047–4051. [Google Scholar] [CrossRef]
- Kampes, R.; Zechel, S.; Hager, M.D.; Schubert, U.S. Halogen bonding in polymer science: Towards new smart materials. Chem. Sci. 2021, 12, 9275–9286. [Google Scholar] [CrossRef]
- Brammer, L.; Mínguez Espallargas, G.; Libri, S. Combining metals with halogen bonds. CrystEngComm 2008, 10, 1712–1727. [Google Scholar] [CrossRef]
- Tepper, R.; Schubert, U.S. Halogen bonding in solution: Anion recognition, templated self-assembly, and organocatalysis. Angew. Chem. Int. Ed. 2018, 57, 6004–6016. [Google Scholar] [CrossRef]
- Präsang, C.; Bruce, D.W. Halogen-bonded liquid crystals. Helv. Chim. Acta 2023, 106, e202300008. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Beer, P.D. Halogen bonding aza-BODIPYs for anion sensing and anion binding-modulated singlet oxygen generation. Chem. Commun. 2024, 60, 7983–7986. [Google Scholar] [CrossRef] [PubMed]
- Sutar, R.L.; Huber, S.M. Catalysis of organic reactions through halogen bonding. ACS Catal. 2019, 9, 9622–9639. [Google Scholar] [CrossRef]
- Piedra, H.F.; Valdés, C.; Plaza, M. Shining light on halogen-bonding complexes: A catalyst-free activation mode of carbon–halogen bonds for the generation of carbon-centered radicals. Chem. Sci. 2023, 14, 5545–5568. [Google Scholar] [CrossRef]
- Petrovskii, S.; Khistiaeva, V.; Paderina, A.; Abramova, E.; Grachova, E. Post-functionalization of organometallic complexes via click-reaction. Molecules 2022, 27, 6494. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Y.; Marion, M.B.; Luna, J.C.H.; Ma, W.; Hu, Y.; Ollivier, C.; Mouriès-Mansuy, V.; Fensterbank, L.; Zhao, F.; et al. Visible light-mediated gold-catalyzed alkynylative cyclization of allenoates with iodoalkynes for the synthesis of β-alkynyl-γ-butenolides. Org. Chem. Front. 2024, 11, 5695–5702. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Graber, S.; Schaffner, S.; Neuburger, M.; Housecroft, C.E.; Constable, E.C. Archetype cationic iridium complexes and their use in solid-state light-emitting electrochemical cells. Adv. Funct. Mater. 2009, 19, 3456–3463. [Google Scholar] [CrossRef]
- Rao, D.S.; Reddy, T.R.; Kashyap, S. Chemoselective and stereospecific iodination of alkynes using sulfonium iodate(i) salt. Org. Biomol. Chem. 2018, 16, 1508–1518. [Google Scholar] [CrossRef]
- Zabarska, N.; Sorsche, D.; Heinemann, F.W.; Glump, S.; Rau, S. Towards ruthenium-based building blocks for CuAAC click reactions: Challenges in generating ruthenium(II) poly-pyridine alkynes. Eur. J. Inorg. Chem. 2015, 2015, 4869–4877. [Google Scholar] [CrossRef]
- Winter, A.; Ulbricht, C.; Holder, E.; Risch, N.; Schubert, U.S. Unusual terpyridines as ligands for novel light-emitting iridium(III) complexes: Synthesis and characterization. Aust. J. Chem. 2006, 59, 773–782. [Google Scholar] [CrossRef]
- Ladouceur, S.; Zysman-Colman, E. A comprehensive survey of cationic iridium(III) complexes bearing nontraditional ligand chelation motifs. Eur. J. Inorg. Chem. 2013, 2013, 2985–3007. [Google Scholar] [CrossRef]
- Luengo, A.; Redrado, M.; Marzo, I.; Fernández-Moreira, V.; Gimeno, M.C. Luminescent Re(I)/Au(I) species as selective anticancer agents for HeLa cells. Inorg. Chem. 2020, 59, 8960–8970. [Google Scholar] [CrossRef] [PubMed]
- Bonfant, G.; Melegari, M.; Balestri, D.; Mezzadri, F.; Marzaroli, V.; Bassanetti, I.; Marchiò, L. Supramolecular assemblies in silver complexes: Phase transitions and the role of the halogen bond. Inorg. Chem. 2020, 59, 4140–4149. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, P.; Witas, K.; Nauroozi, D.; Schmid, M.-A.; Dikmen, E.; Tschierlei, S.; Rau, S. Minimizing side product formation in alkyne functionalization of ruthenium complexes by introduction of protecting groups. Z. Anorg. Allg. Chem. 2020, 646, 842–848. [Google Scholar] [CrossRef]
- Siebert, R.; Schlütter, F.; Winter, A.; Presselt, M.; Görls, H.; Schubert, U.S.; Dietzek, B.; Popp, J. Ruthenium(II)-bis(4′-(4-ethynylphenyl)-2,2′:6′,2″-terpyridine)—A versatile synthon in supramolecular chemistry. Synthesis and characterization. Open Chem. 2011, 9, 990–999. [Google Scholar] [CrossRef]
- Rest, C.; Philips, D.S.; Dünnebacke, T.; Sutar, P.; Sampedro, A.; Droste, J.; Stepanenko, V.; Hansen, M.R.; Albuquerque, R.Q.; Fernández, G. Tuning aqueous supramolecular polymerization by an acid-responsive conformational switch. Chem. Eur. J. 2020, 26, 10005–10013. [Google Scholar] [CrossRef]
- NMRium: NMR Spectra Processing for Everybody. Available online: https://www.nmrium.org (accessed on 1 April 2025).
- Patiny, L.; Musallam, H.; Bolaños, A.; Zasso, M.; Wist, J.; Karayilan, M.; Ziegler, E.; Liermann, J.C.; Schlörer, N.E. NMRium: Teaching nuclear magnetic resonance spectra interpretation in an online platform. Beilstein J. Org. Chem. 2024, 20, 25–31. [Google Scholar] [CrossRef]
- Hooft, R.W.W. COLLECT—Data Collection Software; Nonius B.V.: Delft, The Netherlands, 1998. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology; Academic Press: Cambridge, UK, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Sheldrick, G.M. SADBAS—Version 2.10; Bruker AXS Inc.: Madison, WI, USA, 2022. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]

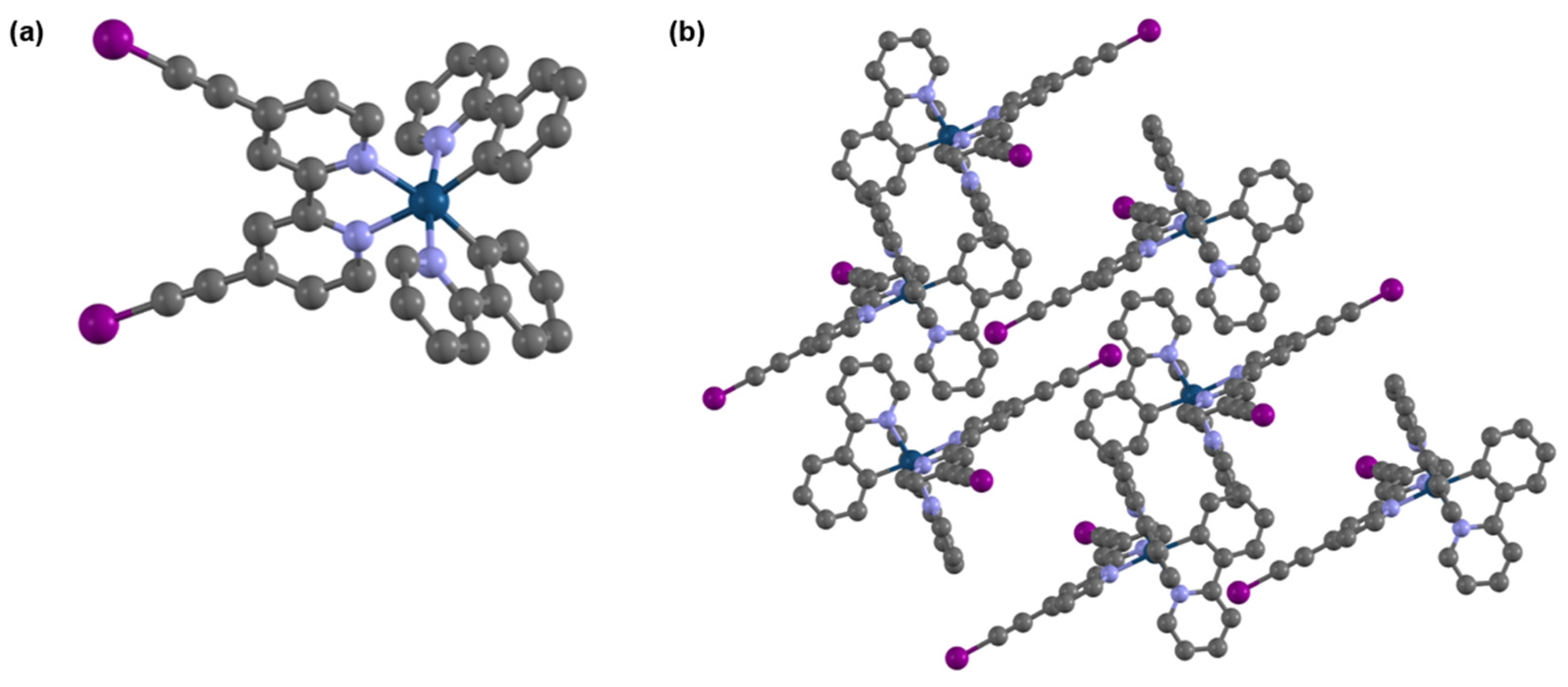
| Bond | Length (Å) | Length (Å) | Bond | Angle (°) | Angle (°) |
|---|---|---|---|---|---|
| Ir–Nbpy | 2.135(5) | 2.132(3) 1 | Nppy–Ir–Nppy | 171.4(3) | 172.1(1) 1 |
| Ir–Nppy | 2.057(5) | 2.044(3) 1 | Nbpy–Ir–Cppy | 171.1(2) | 171.8(1) 1 |
| Ir–Cppy | 2.019(6) | 2.014(4) 1 | 147.9(2) 1 | ||
| C≡C | 1.209(10) | 1.179(8) 2 | Nbpy–Ir–Nbpy | 76.5(3) | 76.2(1) 1 |
| I–C | 1.985(7) | Nppy–Ir–Cppy | 80.2(2) | 80.7(1) 1 | |
| 80.1(1) 1 | |||||
| I–C≡C | 177.8(7) | ||||
| C≡C–Cbpy | 179.0(8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endres, P.; Singh, N.; Winter, A.; Görls, H.; Schubert, U.S. bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate. Molbank 2025, 2025, M2024. https://doi.org/10.3390/M2024
Endres P, Singh N, Winter A, Görls H, Schubert US. bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate. Molbank. 2025; 2025(2):M2024. https://doi.org/10.3390/M2024
Chicago/Turabian StyleEndres, Patrick, Nishi Singh, Andreas Winter, Helmar Görls, and Ulrich S. Schubert. 2025. "bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate" Molbank 2025, no. 2: M2024. https://doi.org/10.3390/M2024
APA StyleEndres, P., Singh, N., Winter, A., Görls, H., & Schubert, U. S. (2025). bis(2-Phenylpyridinato)-[4,4′-bis(iodoethynyl)-2,2′-bipyridine]-iridium(III) Hexafluorophosphate. Molbank, 2025(2), M2024. https://doi.org/10.3390/M2024








