(Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone
Abstract
1. Introduction
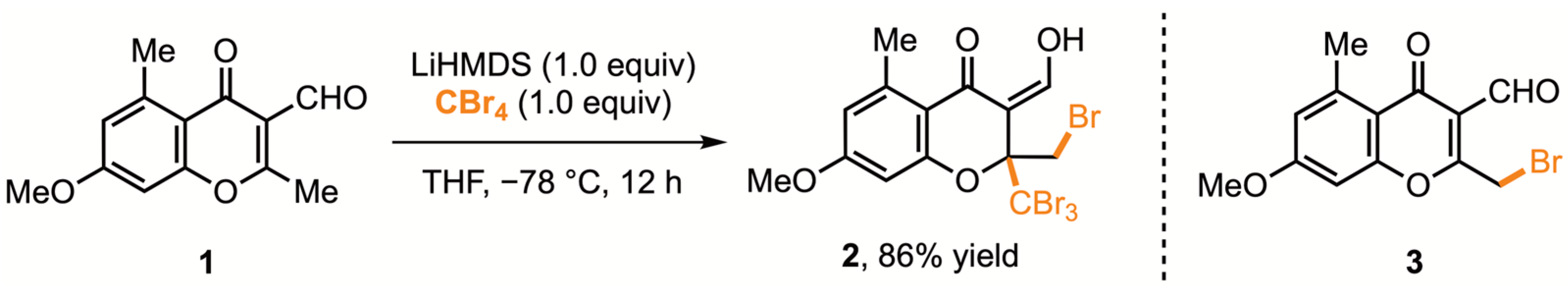
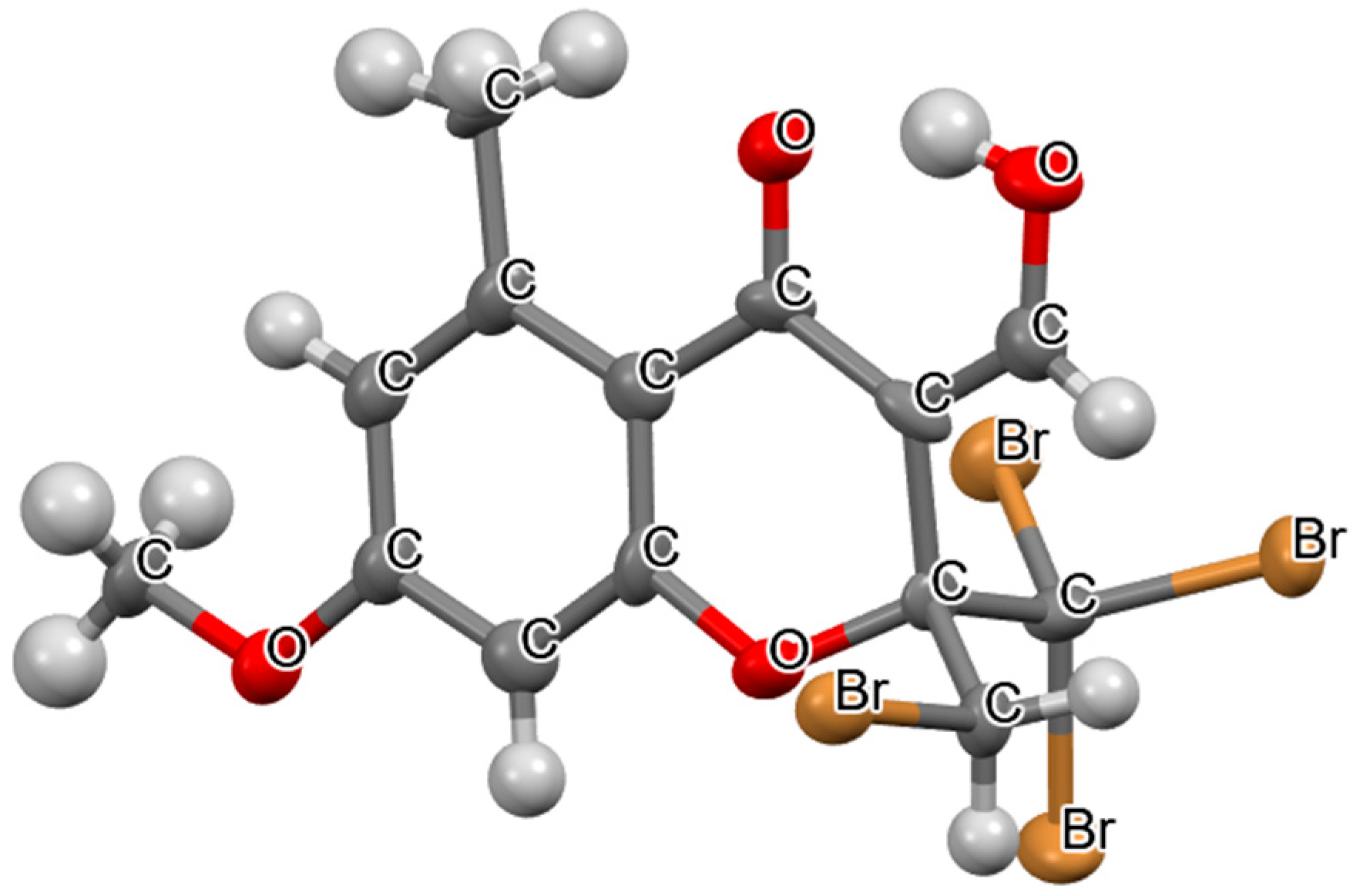
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Information
3.2. Preparation of the Title Compound
3.3. X-Ray Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Saikia, I.; Borah, A.J.; Phukan, P. Use of Bromine and Bromo-Organic Compounds in Organic Synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- Méndez, L.; Salazar, M.O.; Ramallo, I.A.; Furlan, R.L.E. Brominated Extracts as Source of Bioactive Compounds. ACS Comb. Sci. 2011, 13, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Voskressensky, L.G.; Golantsova, N.E.; Maharramov, A.M. Recent Advances in Bromination of Aromatic and Heteroaromatic Compounds. Synthesis 2016, 48, 615–643. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, T.A.; Punniyamurthy, T. Recent Advances in the Application of Tetrabromomethane in Organic Synthesis. Org. Chem. Front. 2021, 8, 4288–4313. [Google Scholar] [CrossRef]
- Bringmann, G.; Feineis, D.; Brückner, R.; Blank, M.; Peters, K.; Peters, E.-M.; Reichmann, H.; Janetzky, B.; Grote, C.; Clement, H.-W.; et al. Bromal-Derived Tetrahydro-β-carbolines as Neurotoxic Agents: Chemistry, Impairment of Dopamine Metabolism, and Inhibitory Effects on Mitochondrial Respiration. Bioorg. Med. Chem. 2000, 8, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Ding, H.; Sun, Z.; Ma, Q.; Yuan, Y.; Jia, X. Halogen Bond (XB) Promoted α-Tribromomethylation of N-Aryltetrahydroisoquinolines and Further Cyclization to 5,6-Dihydroindolo[2,1-a]isoquinolines. J. Org. Chem. 2023, 88, 11310–11321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, C.; Zhao, J.; Liu, G. Rhodium-Catalyzed Cascade Radical Cyclization of 1,6-Enynes with Br–CX3: Access to Bromine-Containing Trihalomethylated Pyrrolidines. Asian J. Org. Chem. 2019, 8, 2249–2256. [Google Scholar] [CrossRef]
- Lee, C.-C.; Shia, K.-S.; Wu, Y.-K. Direct β-Methylation of α-Activated Cross-Conjugated Cycloalkenone Systems: Total Synthesis of Benastatins B and D and Me-oxo-pre-bikaverin. In Proceedings of the 7th NYCU-Gakushuin Student Symposium, Hsinchu, Taiwan, 2–3 November 2024; Abstract O-20. National Yang Ming Chiao Tung University: Hsinchu, Taiwan, 2024. [Google Scholar]
- Baird, M.S.; Buxton, S.R.; Sadler, P. Phase-Transfer Catalysed Reactions of Bromoform with Activated Alkenes: Evidence for a Tribromomethyl Anion Pathway. Tetrahedron Lett. 1985, 26, 6353–6356. [Google Scholar] [CrossRef]
- Hwu, J.R.; Gilbert, B.A. Counterattack Reagents in Organic Reactions and in Syntheses. Tetrahedron 1989, 45, 1233–1261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Wu, Y.-K. (Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone. Molbank 2025, 2025, M2023. https://doi.org/10.3390/M2023
Lee C-C, Wu Y-K. (Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone. Molbank. 2025; 2025(2):M2023. https://doi.org/10.3390/M2023
Chicago/Turabian StyleLee, Chein-Chung, and Yen-Ku Wu. 2025. "(Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone" Molbank 2025, no. 2: M2023. https://doi.org/10.3390/M2023
APA StyleLee, C.-C., & Wu, Y.-K. (2025). (Z)-2-(Bromomethyl)-3-(hydroxymethylene)-7-methoxy-5-methyl-2-(tribromomethyl)-4-chromanone. Molbank, 2025(2), M2023. https://doi.org/10.3390/M2023





