4,4-Dichloro-1,3-dithietane-2-one
Abstract
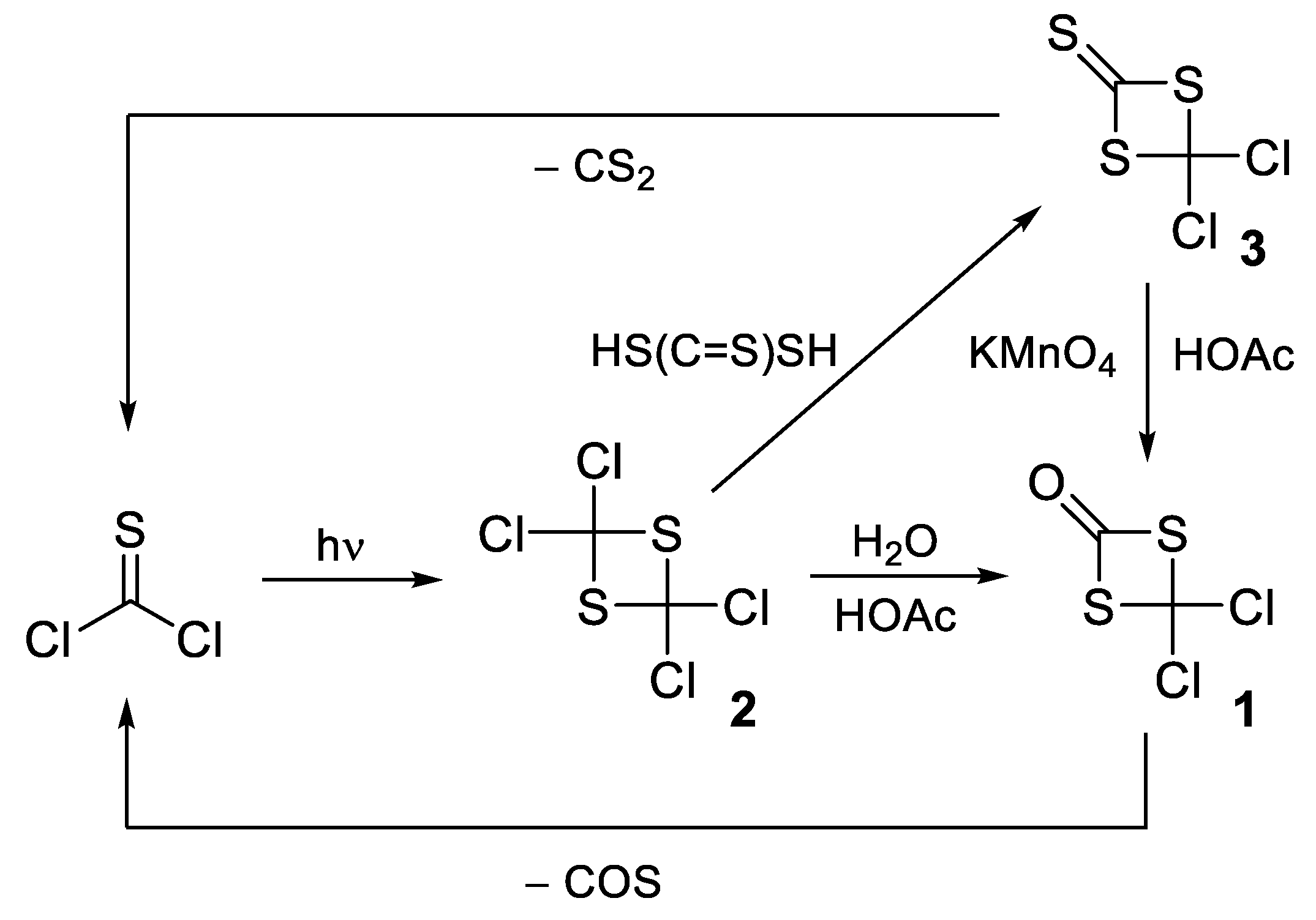
1. Introduction
2. Results and Discussion
2.1. X-Ray Structure
2.2. Additional Characterization of the Long-Term Storage Product
2.3. Mechanistic Possibilities to Account for the Long-Term Storage Product
2.4. Potential Applications and Future Directions
3. Materials and Methods
3.1. General
3.2. Experimental
3.3. X-Ray Data Collection
3.4. X-Ray Structure Solution and Refinement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barany, G. A New Sulfur Heterocycle for Amino Group Protection in Peptide Synthesis. Ph.D. Thesis, The Rockefeller University, New York, NY, USA, 1977. [Google Scholar]
- Barany, G.; Schroll, A.L.; Mott, A.W.; Halsrud, D.A. A General Strategy for Elaboration of the Dithiocarbonyl Functionality,–(C=O)SS–: Application to the Synthesis of Bis(chlorocarbonyl)disulfane and Related Derivatives of Thiocarbonic Acids. J. Org. Chem. 1983, 48, 4750–4761. [Google Scholar] [CrossRef]
- Mott, A.W.; Barany, G. Chlorination of Methylthio(thiocarbonyl) Compounds with Sulfuryl Chloride. Sulfur Lett. 1984, 2, 241–248. [Google Scholar]
- Schroll, A.L.; Barany, G. Novel Symmetrical and Mixed Carbamoyl and Amino Polysulfanes by Reactions of (Alkoxydichloromethyl)Polysulfanyl Substrates with N-Methylaniline. J. Org. Chem. 1986, 51, 1866–1881. [Google Scholar] [CrossRef]
- Kolbe, H. Über die Einwirkung des Chlors auf Schwefelkohlenstoff. Justus Liebigs Ann. Chem. 1843, 45, 41–46. [Google Scholar] [CrossRef][Green Version]
- Dyson, G.M. Thiophosgene. Org. Synth. 1926, 6, 86–91, reprinted in Org. Synth. Coll. 1941, 1, 506–511. [Google Scholar] [CrossRef]
- Rathke, B. Ueber Chlorthioameisensäuremethyläther, das polymere Thiocarbonylchlorid. Ber. Deut. Chem. Ges. 1888, 21, 2539–2545. [Google Scholar] [CrossRef]
- Tilles, H. Thiophosgene. In The Chemistry of Organic Sulfur Compounds; Kharasch, N., Meyers, C.Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1966; pp. 311–336. [Google Scholar] [CrossRef]
- Sharma, S. Thiophosgene in Organic Synthesis. Synthesis 1978, 1978, 803–820. [Google Scholar] [CrossRef]
- Corey, E.J.; Winter, R.A.E. A New, Stereospecific Olefin Synthesis from 1,2-Diols. J. Am. Chem. Soc. 1963, 85, 2677–2678. [Google Scholar] [CrossRef]
- Corey, E.J.; Hopkins, B. A mild procedure for the conversion of 1,2-diols to olefins. Tetrahedron Lett. 1982, 23, 1070–1982. [Google Scholar] [CrossRef]
- Delépine, M.; Labro, L.; Lange, F. Sur le dimère du chlorsulfure de carbone. Sur le chloro-oxy-sulfure C2S2OCl2 et un nouveau chlorosulfure C2S3Cl2 qui en dérivent. Bull. Soc. Chim. Fr. 1939, 2, 1969–1980. [Google Scholar]
- Schöberg, A.; Stephenson, A. Über Die Konstitution Des Photodimeren Thiophosgens. Ber. Deut. Chem. Ges. 1933, 66, 567–571. [Google Scholar] [CrossRef]
- Wortmann, J.; Kiel, G.; Gattow, G. Über Derivate des dimeren Thiophosgens. Z. Naturforsch. B 1968, 23, 1546. [Google Scholar] [CrossRef]
- Wortmann, J.; Kiel, G.; Gattow, G. Über Chalkogenocarbonate. XLI. Derivate des dimeren Thiophosgens. 1. Darstellung und Eigenschaften von SCS2CCl2 und OCS2CCl2. Z. Anorg. Allg. Chem. 1970, 376, 64–72. [Google Scholar] [CrossRef]
- Rakitzis, E.T.; Malliopoulou, T.B. Inactivation of Cathepsin D by Dithiophosgene and by 2,2-Dichloro-1,3-Dithiacyclobutanone. Biochem. J. 1976, 153, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.I.; Kynaston, W.; Hales, J.L. The Structure of the Dimer of Thiocarbonyl Chloride and Its Hydrolysis Product. Infrared Absorption Data on Some Compounds Containing the Thiocarbonyl Group. J. Chem. Soc. 1957, 1957, 614–618. [Google Scholar] [CrossRef]
- Krebs, B.; Beyer, H. Kristall- und Molekülstruktur des dimeren Thiophosgens. Zur Bildung und zur Struktur von 2.2-Dichloro-1.3-dithiacyclobutanon-(4). Z. Naturforsch. B 1968, 23, 741–742. [Google Scholar] [CrossRef]
- Boese, R.; Bläser, D.; Stellberg, P. Crystal Structure of 1,1,3,3-Tetrachloro-2,4- Dithiacyclobutane, (Thiophosgene Dimer), C2Cl4S2. Z. Kristallogr. Cryst. Mater. 1993, 206, 308–309. [Google Scholar] [CrossRef]
- Lin, X.; Huang, Y.; Min, L.; Li, C.-C. Synthesis of Spirotricyclic Core of Bonnadiene. Org. Lett. 2023, 25, 1156–1160. [Google Scholar] [CrossRef]
- Onyanchaa, D.; Nyamorib, V.; McClelanda, C.W.; Imriea, C.; Gerber, T.I.A. Solvent-free reactions of N,N’-thiocarbonyldiimidazoles with ferrocenylcarbinols. J. Organometal. Chem. 2009, 694, 207–212. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Nakagawa, H.; Eto, M.; Harano, K. An X-ray Crystallographic Study of Intermolecular Edge-to-Face Aromatic Interaction in Crystal Structure of exo [4+2]π Cycloadduct of Phencyclone and S-Allyl S-Methyl Dithiocarbonate. Tetrahedron 2000, 56, 6015–6021. [Google Scholar] [CrossRef]
- Musozoda, M.; Muller II, J.E.; Anderson, G.I.; Boucher, M.; Zeller, M.; Raymond, C.C.; Hillesheim, P.C.; Mirjafari, A. Alkyl-templated cocrystallization of long-chain 1-bromoalkanes by lipid-like ionic liquids. Chem. Commun. 2024, 60, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Waterfeld, A.; Mews, R. Fluor-Halogen-Austausch an 2,2,4,4-Tetrafluor-1,3-dithietan. Chem. Ber. 1985, 118, 4997–5005. [Google Scholar] [CrossRef]
- Taylor, E.C.; Poole, J.P. A Method for Evaluating the Light Protection of Amber Glass. Proc. Annu. Meet. Am. Soc. Brew. Chem. 1971, 29, 238–245. [Google Scholar] [CrossRef]
- Seddon, E.A.; Ryan, C.; Seddon, K.R.; Ryan, T.A. Phosgene substitutes and homologues. In Phosgene; Elsevier Science & Technology: Amsterdam, The Netherlands, 1996; Volume 24. [Google Scholar]
- Eckert, H.; Forster, B. Triphosgene, a Crystalline Phosgene Substitute. Angew. Chem. Int. Ed. Engl. 1987, 26, 894–895. [Google Scholar] [CrossRef]
- Cotarca, L.; Geller, T.; Répási, J. Bis(Trichloromethyl)Carbonate (BTC, Triphosgene): A Safer Alternative to Phosgene? Org. Process Res. Dev. 2017, 21, 1439–1446. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- APEX3, Version 2015.9-0; Bruker AXS: Madison, WI, USA, 2015.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- SAINT, Version 8.34A; Bruker AXS: Madison, WI, USA, 2013.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]

| Empirical formula | C2Cl2OS2 |
| Formula weight | 175.04 |
| Temperature | 100.0(5) K |
| Wavelength | 0.71073 Å |
| Crystal system | monoclinic |
| Space group | P21/n |
| Unit cell dimensions | a = 5.8312(5) Å, α = 90° |
| b = 10.3605(9) Å, β = 94.4572(17)° | |
| c = 9.3858(8) Å, γ = 90° | |
| Volume | 565.32(8) Å3 |
| Z | 4 |
| Density (calculated) | 2.057 g/cm3 |
| Absorption coefficient | 1.752 mm−1 |
| F(000) | 344 |
| Crystal color, morphology | colorless, block |
| Crystal size | 0.45 × 0.40 × 0.30 mm3 |
| Theta range for data collection | 2.933 to 38.651° |
| Index ranges | −10 ≤ h ≤ 10, −18 ≤ k ≤ 18, −16 ≤ l ≤ 16 |
| Reflections collected | 41,413 |
| Independent reflections | 3169 [R(int) = 0.0216] |
| Observed reflections | 3058 |
| Completeness to θ = 37.785° | 99.7% |
| Absorption correction | Multi-scan |
| Max. and min. transmission | 0.7476 and 0.5909 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3169/0/64 |
| Goodness-of-fit on F2 | 1.183 |
| Final R indices [I > 2σ(I)] | R1 = 0.0216, wR2 = 0.0549 |
| R indices (all data) | R1 = 0.0226, wR2 = 0.0554 |
| Largest diff. peak and hole | 0.507 and −0.400 e−/Å3 |
| 1 | 2 a | Non-Cyclic | |
|---|---|---|---|
| S1–C2 | 1.8420(7) | 1.801(6) | |
| S1–C4 | 1.7825(7) | 1.76 | |
| S3–C2 | 1.8229(7) | 1.807(6) | |
| S3–C4 | 1.7764(7) | ||
| C2–Cl1A | 1.7745(7) | 1.760(6) | |
| C2–Cl2A | 1.7748(7) | 1.778(6) | |
| S1–C2–S3 | 96.62(3) | 96.1(6) | |
| S1–C4–S3 | 99.86(3) | 110.9 | |
| C2–S1–C4 | 81.65(3) | 83.9(5) b | |
| C2–S3–C4 | 81.85(3) | ||
| Cl1A–C2–Cl2A | 108.92(4) | 107.1(4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, T.R.; Brennessel, W.W.; Goebel, E.S.; Turcotte, M.J.; Barany, G. 4,4-Dichloro-1,3-dithietane-2-one. Molbank 2025, 2025, M2021. https://doi.org/10.3390/M2021
Thompson TR, Brennessel WW, Goebel ES, Turcotte MJ, Barany G. 4,4-Dichloro-1,3-dithietane-2-one. Molbank. 2025; 2025(2):M2021. https://doi.org/10.3390/M2021
Chicago/Turabian StyleThompson, Tracy R., William W. Brennessel, Erik S. Goebel, Matthew J. Turcotte, and George Barany. 2025. "4,4-Dichloro-1,3-dithietane-2-one" Molbank 2025, no. 2: M2021. https://doi.org/10.3390/M2021
APA StyleThompson, T. R., Brennessel, W. W., Goebel, E. S., Turcotte, M. J., & Barany, G. (2025). 4,4-Dichloro-1,3-dithietane-2-one. Molbank, 2025(2), M2021. https://doi.org/10.3390/M2021






