Abstract
Black, needle-like single crystals of [Pd@Bi10][AlCl4]4 were synthesized in a one-pot reaction between PdCl2, Bi, and BiCl3 at 180 °C in the Lewis acidic ionic liquid (LAIL) medium [BMIm]Cl∙4.2AlCl4 (BMIm = 1-n-butyl-3-methylimidazolium). Single-crystal X-ray diffraction revealed that the compound crystallizes in the triclinic space group P with the unit cell parameters a = 11.0233(5) Å, b = 26.1892(14) Å, c = 26.2687(14) Å, α = 90.842(2)°, β = 92.1940(10)°, γ = 91.164(2)°, closely matching its platinum-containing analog. The structure features pentagonal antiprismatic [Pd@Bi10]4+ cluster cations charge-balanced by tetrahedral [AlCl4]− anions. Bonding and charge analysis reveal unoptimized Pd–Bi and strong Bi–Bi covalent interactions consistent with electronegativity trends and the previously reported host–guest model. Electronic structure calculations performed with the TB-LMTO-ASA program show that [Pd@Bi10][AlCl4]4 exhibits semiconducting behavior, suggesting a bandgap opening of 0.71 eV.
1. Introduction
Halometallate ionic liquids (ILs) are extensively researched room-temperature molten salts formed by the reaction of a metal halide with an organic halide salt [1,2]. These compounds typically melt at temperatures below 100 °C, enhancing the solubility of various reactants in ILs and promoting reactions at low temperatures, which, in turn, enables green chemical processes and provides a uniform reaction environment [3]. Although ILs have long been applied in organic chemistry, their intensive use in inorganic synthesis emerged in the early 21st century, allowing the discovery of numerous inorganic compounds and materials that were inaccessible by traditional synthetic methods such as high-temperature solid-state and solvothermal reactions [4]. ILs possess unique properties that distinguish them from conventional organic solvents, classic melts, or solid-state metal flux systems. These include a wide liquidus range, high redox and thermal stability, negligible vapor pressure, and adjustable polarity, to name a few [1]. Consequently, ILs have found uses in lubrication, electrodeposition, electrolytes in photovoltaic devices, clean catalysis, polymerization, the general synthesis of novel inorganic compounds, and, most notably, crystal engineering of diverse inorganic materials [5,6,7,8,9,10].
Over the past two decades, bismuth cluster compounds have been extensively studied due to their non-toxic and non-radioactive nature, as well as their significant potential as catalysts for novel pharmaceuticals and fine-chemical synthesis. ILs hold great promise for advancing bismuth chemistry, offering low-temperature routes for the synthesis of Bi-bearing clusters [11]. Particularly, Lewis acidic ILs (LAILs) such as [BMIm]TrCl4 (BMIm = 1-n-butyl-3-methylimidazolium; Tr = Al, Ga, In) have emerged as versatile media for the low-temperature synthesis of homoatomic Bi cluster compounds including [Bi5][AlCl4]3, [Bi5][GaCl4]3, Bi8[AlCl4]2, and Bi8[GaCl4]2, to name a few [12,13,14]. Heterometallic Bi clusters in which electron-rich transition metals serve as guests within the Bi cluster host have likewise been discovered by introducing transition metal chloride salts into LAILs [15]. A few notable examples are [CuBi8][AlBr4]2[Al2Br7] [15], [M@Bi10][AlBr4]4 [16], [M@Bi10][AlBr4]2[Al2Br7]2 (M = Pd, Pt) [16], [Pd@Bi10][Bi2Sn6Cl22] [16], [Ni2Bi12][AlCl4]3[Al2Cl7] [17], [Rh2Bi12][AlBr4]4 [17], [Ru2Bi14Br2][AlCl4]4 [18], AuBi13.6Sn2.4Cl20.6 [19], and [Bi8]Tl[AlCl4]3 [20], where “@” denotes the encapsulation of a transition metal within the cluster polycation, such as [Bi10]4+. The broad applicability of ILs in inorganic cluster synthesis is further exemplified by the recent discovery of numerous non-Bi heterometallic cluster compounds, including [Mo2Te12]I6 [21], Te6[WOCl4]2 [21], and [Sb7Se8Br2][AlX4]3·NbCl5 (X = Cl, Br) [22], to name a few.
Particularly interesting is the family of compounds containing [Bi10]4+ polycation, which can serve as a host for electron-rich heavy metals, such as Pt, Pd, and Au [15,16,23,24,25,26], or as a linking unit in more complex molecules, such as [Au2Bi10](PnBi3Br9)2 (Pn = As, Sb, Bi) [27]. Three pentagonal antiprismatic intermetalloid clusters, [M@Bi10]n+ (M = Pt, Pd, Au; n= +4/+5), were synthesized in LAILs at near-room temperature [15,16]. These clusters obey the Wade–Mingos electron-counting rules and are classified as arachno-type clusters [28,29]. In all cases, these cluster cations are charge-balanced by [AlX₄]− (X = Cl, Br) tetrahedral anions.
In this paper, we report the one-pot synthesis, comprehensive structural characterization, and details of the electronic structure of the novel [Pd@Bi10][AlCl4]4 phase, extending the family of known cluster compounds containing the [Bi10]4+ structural unit. Although this compound has been previously identified [15], its structural characterization could not be completed due to poor crystal quality. However, through the use of high-quality single crystals and an extended exposure time, we have successfully solved the crystal structure of [Pd@Bi10][AlCl4]4 and performed electronic structure calculations, providing insightful details regarding chemical bonding within the heterometallic Bi-bearing clusters.
2. Results and Discussion
2.1. Crystal Structure
The family of compounds containing [M@Bi10]4+ clusters is compositionally and structurally abundant, vide supra [16,19]. Such structural diversity is particularly pronounced within isoelectronic [M@Bi10][AlX4]4 (M = Pd, Pt; X = Cl, Br) compositions relevant to the title compound. For instance, [Pd@Bi10][AlBr4]4 crystallizes in the tetragonal space group P42/n (ICSD 431980), whereas the Pt-bearing analog [Pt@Bi10][AlBr4]4 exists in two polymorphic modifications, the high-temperature form P42/n (ICSD 431978) and the low-temperature form P (ICSD 431977) [16]. In contrast, the chloride analog [Pt@Bi10][AlCl4]4 exhibits lower symmetry and crystallizes in the triclinic space group P (ICSD 124212) [15]. Similarly, the title [Pd@Bi10][AlCl4]4 phase is isostructural to the Pt-containing analog and adopts the same triclinic archetype (Table 1, Table S1 and Table S2).

Table 1.
Selected crystallographic data collection details and structure refinement details for [PdBi10][AlCl4]4.
The crystal structure of the monoclinic [M@Bi10][AlX4]4 polymorph is well established [15]; therefore, we provide only a brief description. The unit cell comprises four crystallographically unique [Pd@Bi10]4+ polycations charge-balanced by 16 [AlCl4]− anions (Figure 1a). A typical polycation bears a pentagonal antiprismatic structure featuring a [Bi10]4+ cage that encapsulates an endohedral Pd atom, similar in nature to the previously reported [Pt@Bi10][AlX4]4 [16,19]. According to the Wade–Mingos rules, the [Bi10]4+ shell is classified as an arachno-cluster with 26 skeletal electrons (N.B., only 6p electrons of Bi are considered to participate in skeletal bonding [28,29]). This corresponds to 13 electron pairs, which satisfies the n + 3 rule (where n = 10 cluster vertices), consistent with electron requirements for an arachno-type cluster.
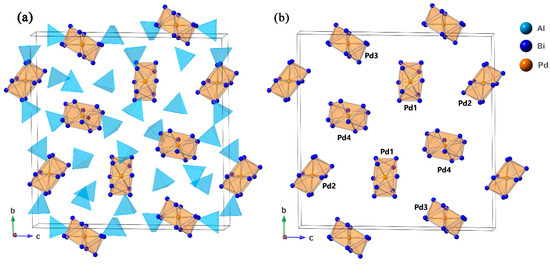
Figure 1.
(a) A schematic representation of [PdBi10][AlCl4]4 unit cell with both [Pd@Bi10]4+ polycations, drawn in orange, and [AlCl4]− anions, drawn in sky-blue. (b) Packing of the polycations within the unit cell, showing four crystallographically independent cluster units. [AlCl4]− anions are omitted for clarity. The unit cell is outlined, Pd atoms are orange, Bi atoms are blue, and Al atoms are sky-blue.
The four crystallographically unique [Pd@Bi10]4+ polycations are labeled as Pd1, Pd2, Pd3, and Pd4 in Figure 1b. Each formally neutral Pd atom exhibits tenfold coordination by surrounding Bi atoms, which is viewed as the maximum attainable CN in this framework, and is a hallmark of [M@Bi10]n+-type cationic cluster compounds [15,16,19]. Within each cluster, every Bi atom is coordinated to four neighboring Bi atoms (two within the pentagonal face and two within the triangular face) and one Pd atom, resulting in a fivefold coordination environment. The pentagonal bases of the [Bi10]4+ antiprisms are parallel to each other, with an approximate distance of 2.55 Å between planes.
The symmetric nature and structural identity of the four crystallographically unique clusters are evident from the examination of bond lengths and bond angles (Figure 2, Table S2). The Pd–Bi bond lengths are in the range of 2.9636(10)–3.0152(11) Å for Pd1–Bi, 2.9573(11)–3.0083(11) Å for Pd2–Bi, 2.9364(10) –3.0098(10)Å for Pd3–Bi, and 2.9449(11)–3.0236(10) Å for Pd4–Bi, consistent with those of previously reported [M@Bi10]4+ (M = Pt, Pd) clusters [15,16], though slightly longer than the sum of the covalent radii of Pd and Bi. The Bi–Bi bond lengths at the base of the cluster span from 3.1168(7) to 3.1964(7) Å, with the largest distribution for the Pd1-based cluster (Table S2) and are in good agreement with those observed in related [M@Bi10]n+ clusters and in the [Bi8]2+ fragment [13,14,15,16]. The triangular faces of the [Bi10]4+ unit exhibit slightly shorter average Bi–Bi distances, ranging from 3.0656(9) to 3.1552(9) Å, with the largest distribution for the Pd4-based cluster, although these observations are in good agreement with previous reports, the results of our calculations (vide infra), and the expected interatomic distances for homoatomic Bi–Bi contacts in Zintl bismuthides [30,31]. The Bi–Bi–Bi bond angles within the pentagonal base vary from 105.08(2)° to 110.87(2)°, with the largest distribution for the Pd4-based cluster, closely approximating the ideal pentagonal angle of 108° (Table S2). Please note that the angles for the other three clusters are within the same range, but less widely distributed, being closer to 108° (Table S2). The respective angles within the triangular face are close to 60°, as expected for an equilateral triangle (Table S2). It is important to note that the four clusters in the isostructural [PtBi10][AlCl4]4 phase are also symmetrically identical, but possess minimal discrepancy within the Pt–Bi and Bi–Bi bonding, comparable to the discussed title compound [15].
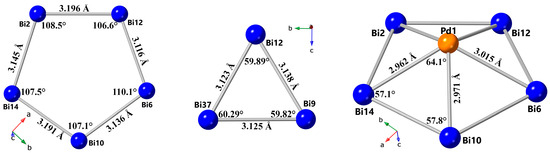
Figure 2.
The Bi–Bi bond distances and bond angles of the pentagonal base (a) and triangular face (b), and the Bi–Pd bond lengths and bond angles of the [Pd1@Bi10]4+ cluster (c).
The Al–Cl bond lengths fall between approximately 2.115 and 2.156 Å, within the expected range for Al–Cl bonding and comparable to those in reported Bi-bearing clusters with the [AlCl4]ˉ counter anion [12,14]. The observed Cl–Al–Cl bond angles show only minimal deviation from the ideal tetrahedral angle of 109.4°, consistent with sp3 hybridization of the Al atoms, as further discussed in the following section.
2.2. Electronic Structure
Following single-crystal XRD analysis, DFT calculations were performed for the [Pd@Bi10]4+ polycation using various exchange-correlation functionals within the TURBOMOLE program. Geometry-optimized interatomic distances and bond angles were compared with the experimental values (Table 2) to validate the accuracy of the calculated structural model. The computational results showed good agreement with the experimental data across all the tested functionals. Notably, the closest correspondence was observed for calculations employing the COSMO solvation method with TPSSh functional.

Table 2.
Selected interatomic distances of the optimized equilibrium geometry of the [Pd@Bi10]4+ polycation, as obtained in the def2-TZVP basis using different DFT functionals.
The Electron Localization Function (ELF) analysis of the geometry-optimized [PdBi10]4+ cluster reveals a uniform degree of electron localization across the bases and faces of the cluster. The ELF isosurface maps (Figure 3a) further highlight distinct bonding interactions among the Bi atoms with clear localized domains between atomic pairs, illustrating the symmetric nature of the cluster [32,33]. On the other hand, we did not observe significant localized domains between Pd and Bi atoms, which corroborates the previously observed host–guest nature of the bonding within the [M@Bi10]4+ polycation. The HOMO–LUMO energy gaps for the cluster, computed using different functionals, are 2.31 eV (TPSSh), 4.31 eV (M06-2X), and 2.69 eV (B3-LYP), indicating functional-dependent variation in the predicted electronic properties. However, all the values are relatively large, implying the stability of the structural fragment.
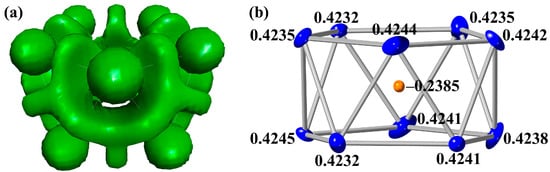
Figure 3.
(a) The ELF isosurface (η = 0.5) inside the [Pd@Bi10]4+ cluster, calculated with TPSSh functional. (b) The calculated charges of each atom in the cluster, according to the Natural Bond Orbital (NBO) calculations (Pd denoted in orange and Bi in blue). The thermal ellipsoids are plotted at the 50% probability level.
To better understand the interactions within the [PdBi10]4+ cluster, Natural Population Analysis (NPA) was performed via Natural Bond Orbital (NBO) calculations using the TURBOMOLE program, based on the geometry optimized with the TPSSh functional using the COSMO solvation method. The effective charges were calculated for all the atoms in the cluster (Table S3 and S4). The more electronegative Pd atom (EN = 2.28) carries a partial negative charge of ca. –0.24, while all the Bi atoms (EN = 2.02) exhibit positive charges of ca. +0.42 (Figure 3a). These results corroborate well with the notation that the negatively charged Pd center stabilizes the cluster through electron donation, whereas the positively charged Bi atoms reflect a partial depletion of electron density. The charge distribution is consistent with previous reports in which the NPA charges of Pd and Bi were calculated as −0.5 and +0.45, respectively [19]. Such charge separation is indicative of a polarized bonding framework centered around the endohedral Pd, as opposed to a fully delocalized metallic bonding environment, which indicates the covalent nature within the cluster framework.
However, the nature of the bonding between Pd and Bi within the [PdBi10]4+ cluster remains debatable. Early reports suggested a host–guest scenario with negligible bonding interaction between the encapsulated metal and the Bi10 framework [16,19], which was also observed in the ELF analysis described above. On the other hand, more recent ELF analyses of multiple clusters have indicated non-negligible Pd–Bi bonding interactions [34].
To further investigate the electronic structure of the [Pd@Bi10][AlCl4]4 phase and assess orbital overlap, we performed density of states (DOS) and crystal orbital Hamilton population (COHP) calculations using the TB-LMTO-ASA program. The results are presented in Figure 4. Major contributions to the valence band maxima (VBM) are provided by the Pd d, Bi p, and, to a lesser extent, Cl p orbitals. Notably, there is a clear overlap between the Pd d and Bi p states in the region just above –2.2 eV, which may be indicative of Pd–Bi interaction within the polycation. These contacts show a combination of bonding (between −2 eV and −0.9 eV) and antibonding (−0.9 eV to VBM) states below the Fermi level (Figure 4f). Pd–Bi bonding is quantified with the calculated −ICOHP values of ca. 0.92 eV/bond, which is indicative of a weak interaction, as opposed to the calculated −ICOHPs of ca. 2.43 eV/bond for strong covalent Nb–As bonding within the [NbAs10]5− cluster [33].
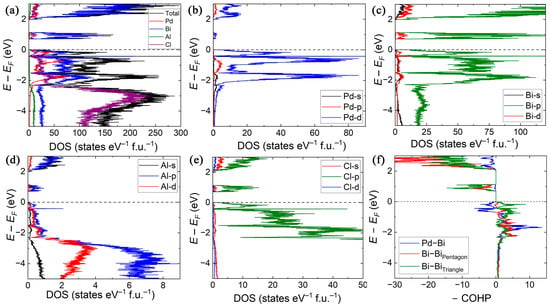
Figure 4.
The calculated (a) total density of states (TDOS) of [Pd@Bi10][AlCl4]4 and partial density of states (PDOS) for Pd (b), Bi (c), Al (d), and Cl (e); (f) the calculated COHP curves for Bi–Pd heteroatomic bonds and Bi–Bi bonds. The Fermi level is set as the reference point at 0 eV and is dashed.
The overall dominance of the Bi 6p states in the valence band is consistent with the Bi-rich composition of the title compound. Interestingly, the minimal contribution of the Bi 6s orbitals supports the presence of localized lone pairs and suggests poor s–p orbital overlap. In contrast, the Al s, p, and d orbitals overlap in the same energy window below −2 eV; thus, averaging their energies suggests a perfect symmetry with the nearly ideal tetrahedral geometry of the [AlCl4]− anions. This is further supported by the significant contribution of the Cl p states in the same energy range. The conduction band minima (CBM) is predominantly derived from the Bi p states.
Before we discuss the details of Bi–Bi interactions within the cluster, we want to reiterate that these bonds can be classified within pentagonal bases and triangular faces (Figure 2). Although all of these bonds are optimized at the Fermi level (Figure 4f), negligible antibonding domains are observed for Bi–Bi interactions within the pentagon down to −0.4 eV. In combination with the slightly longer bond distances, compared to Bi–Bi bonds within the triangular faces (Table S2), this may be an indication of slightly weaker covalent interactions. Indeed, the average −ICOHP values for (Bi–Bi)triangle and (Bi–Bi)pentagon are –3.8 eV/bond and –2.25 eV/bond, respectively, which confirms a more pronounced covalent nature of the former.
Electronic structure calculations reveal that [Pd@Bi10][AlCl4]4 exhibits semiconducting behavior with a calculated bandgap of 0.71 eV, which is consistent with the structurally related cluster compound [Pd@Bi10][Bi2Sn6Cl22], which features a comparable bandgap of 0.9 eV and similarly displays a black appearance [16,25].
3. Materials and Methods
3.1. Synthesis
Commercially available starting materials, anhydrous PdCl2 (Pressure Chemicals Co., Pittsburgh, PA, USA, 60% Pd)) and elemental Bi (Sigma-Aldrich, 99.99%), were used as received. AlCl3 (Thermo Scientific, Waltham, MA, USA, 99%) was dried at 35 °C for 48 h, and [BMIm]Cl (Alfa Aesar, Waltham, MA, USA, 96%) was vacuum dried at 130 °C for 48 h before use. Anhydrous BiCl3 (Thermo Scientific, Waltham, MA, USA, 99.9%) was sublimated prior to use.
All synthetic and post-synthetic manipulations were carried out in an argon-filled glovebox with O2 level and H2O level less than 1 ppm. The synthesis was performed in a vacuum-sealed quartz ampule with a diameter of 14 mm and a length of 100mm. PdCl2 (14.2 mg), Bi (153.3 mg), AlCl3 (450 mg), [BMIm]Cl (150 mg), and BiCl3 (21.0 mg) were added to the ampule, wherein the IL mixture began to liquefy immediately, yielding a light-brown solution. The sealed ampule was placed in a slightly tilted tube furnace to ensure that all the reaction materials settled at the bottom. The reaction mixture was maintained at 180 °C for 48 h and then cooled to room temperature at the rate of 6 °C/h, consistent with a previous report [16]. Upon completion, black, needle-shaped crystals of [Pd@Bi10][AlCl4]4 were obtained. These crystals were found to be air-sensitive, which complicated further characterization of their properties. As a minor byproduct, red-colored, cube-shaped crystals of Bi5[AlCl4]3 were identified [12]. The two phases were easily distinguished under a microscope.
3.2. Single Crystal X-Ray Diffraction
The crystal structure of [Pd@Bi10][AlCl4]4 was investigated using the Single Crystal X-ray Diffraction (SCXRD) method. An Ag Kα (λ = 0.56086 Å) radiation source was used to capture data on a Bruker D8 Venture DUO, with a Photon III C14 detector diffractometer, under a cold nitrogen stream at 100(2) K. Suitable single crystals were chosen, sliced to the required dimensions of under 100 μm, and mounted on MiTeGen plastic loops. Data integration and absorption correction were performed using the SAINT and SADABS programs (v.8.40B), respectively, both implemented with the APEX4 software suite (v2021.4.0) [35,36]. A multi-scan absorption correction was applied, yielding minimum and maximum transmission factors of 0.1447 and 0.2535, respectively. The crystal structure was solved using the SHELXT program (v.2018/2) and refined with the SHELXL program (v.2018/1), using Olex2-1.5 as a graphical interface [37,38,39]. An isotropic extinction correction was applied during the refinement, yielding a refined extinction coefficient of 0.000036(2). Atomic coordinates and labels were standardized using the STRUCTURE TIDY program [40]. Structural illustrations were prepared using the CrystalMaker software (v.11.0.1) [41].
3.3. Electronic Structure Calculations
Electronic structure calculations were performed using two complementary approaches. First, molecular quantum chemical calculations were performed on the isolated [Pd@Bi10]4+ polycation using the TURBOMOLE program v.7.8 [42]. Density Functional Theory (DFT) computations were performed with TPSSh, M06-2X, and B3-LYP functionals including Effective Core Potential (def2-ECP) with def2-TZVP basis [43,44,45,46,47]. The COSMO solvation method was used in the TPSSh functional with relative permittivity ε = 100 [48]. Geometry optimizations were performed with all the functionals with tight thresholds for the energy and gradient (scfconv = 7) [49]. Electron Localization Function (ELF) analysis and Natural Population Analysis (NBO) were conducted at the TPSSh level with the COSMO solvation method [50,51]. Molecular visualizations were generated using the TmoleX 2024 package [52].
Second, density of states (DOS) calculations for the [Pd@Bi10][AlCl4]4 structure were performed at the DFT level using the TB-LMTO-ASA code [53]. The standardized experimental unit cell parameters (Table 1) and atomic coordinates (Table S1) were used without structure optimization. The atomic sphere approximation (ASA) was satisfied by introducing empty spheres, and the von Barth–Hedin functional was employed [54]. After self-consistent convergence, a 17 × 7 × 7 k-point mesh was employed to sample the Brillouin zone. The Fermi level was set to EF = 0 eV. A basic set of orbitals included Pd [5s, 5p, 4d, and (4f)], Bi [6s, 6p, (6d), (5f)], Al [3s, 3p, (3d)] Cl [(4s), 3p, (3d)], with the downfolded orbitals treated with the Löwdin downfolding method given in parentheses. Chemical bonding was studied using crystal orbital Hamilton population (COHP) curves using the module within the TB-LMTO-ASA code [55].
4. Conclusions
In this work, we successfully reproduced and structurally characterized a novel intermetallic Bi cluster compound, [Pd@Bi10][AlCl4]4, via a one-pot reaction using the LAIL [BMIm]·4.2[AlCl4]4. This charge-balanced compound comprises 0D pentagonal antiprismatic [Pd@Bi10]4+ polycations stabilized by isolated [AlCl4]− tetrahedral anions. The symmetric nature of the cluster was confirmed through detailed structural analysis and further validated by DFT calculations. Analysis of the electronic structure revealed non-negligible Pd–Bi bonding interactions, although consistent with the host–guest model, and a charge distribution consistent with electronegativity trends. Further electronic structure calculations performed with the TB-LMTO-ASA code indicated semiconducting behavior with a bandgap of 0.71 eV. This work broadens the scope of [M@Bi10]4+ cluster chemistry and supports prior findings on the viability of LAILs in stabilizing complex intermetalloid clusters under mild conditions, while also providing new insights into covalent transition metal–pnictogen interactions.
Supplementary Materials
Table S1: Refined atomic coordinates and equivalent displacement parameters (Ueq, Å2) for [PdBi10][AlCl4]4; Table S2: Selected interatomic distances (Å) and bond angles (°) in crystallographically independent [PdBi10]4+ cationic clusters; Table S3: Atomic populations from total density calculation via TURBOMOLE program with TPSSh functional and def2-TZVP basis via COSMO solvation method; and Table S4: Natural Bond Orbital (NBO) calculation via TURBOMOLE program with TPSSh functional and def2-TZVP basis via COSMO solvation method.
Author Contributions
Conceptualization, methodology, validation, formal analysis, data curation: S.M.G.K.S. and S.B.; investigation, writing—original draft preparation, visualization: S.M.G.K.S.; resources, writing—review and editing, supervision, project administration, funding acquisition: S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the College of Science and Department of Chemistry at Louisiana State University. X-ray crystallographic data were collected by the diffractometer funded by the NSF MRI award CHE-2215262.
Data Availability Statement
The Crystallographic Information File (CIF) corresponding to the work has been uploaded to the Cambridge Crystallographic Data Center (CCDC) and can be freely obtained by visiting https://www.ccdc.cam.ac.uk/structures/ (access date 1 May 2025) (depository number is 2448552).
Acknowledgments
The authors acknowledge the College of Science and Department of Chemistry at Louisiana State University for the financial support.
Conflicts of Interest
Authors declare no conflicts of interests.
References
- Kore, R.; Kelley, S.P.; Sawant, A.D.; Mishra, M.K.; Rogers, R.D. Are ionic liquids and liquid coordination complexes really different?—Synthesis, characterization, and catalytic activity of AlCl3/base catalysts. Chem. Commun. 2020, 56, 5362–5365. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Baaqel, H.; Matthews, R.P.; Chen, Y.; Lovelock, K.R.J.; Hallett, J.P.; Licence, P. Halometallate ionic liquids: Thermal properties, decomposition pathways, and life cycle considerations. Green Chem. 2022, 24, 5800–5812. [Google Scholar] [CrossRef]
- Freudenmann, D.; Wolf, S.; Wolff, M.; Feldmann, C. Ionic liquids: New perspectives for inorganic synthesis? Angew. Chem. Int. Ed. 2011, 50, 11050–11060. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of Inorganic Materials Using Ionic Liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Breternitz, J.; Groh, M.F.; Ruck, M. Ionic liquids as crystallization media for inorganic materials. CrystEngComm. 2012, 14, 4874–4885. [Google Scholar] [CrossRef]
- Dupont, J.; Leal, B.C.; Lozano, P.; Monteiro, A.L.; Migowski, P.; Scholten, J.D. Ionic Liquids in Metal, Photo-, Electro-, and (Bio) Catalysis. Chem. Rev. 2024, 124, 5227–5420. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, C.; Sun, L.; Hu, Y.; Lyu, H.; Wang, Z.; Song, Z.; Yu, G. Energy Applications of Ionic Liquids: Recent Developments and Future Prospects. Chem. Rev. 2023, 123, 12170–12253. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef]
- Baca, K.R.; Al-Barghouti, K.; Wang, N.; Bennett, M.G.; Matamoros Valenciano, L.; May, T.L.; Xu, I.V.; Cordry, M.; Haggard, D.M.; Haas, A.G.; et al. Ionic Liquids for the Separation of Fluorocarbon Refrigerant Mixtures. Chem. Rev. 2024, 124, 5167–5226. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Hallett, J.; Shiflett, M. Introduction: Ionic Liquids for Diverse Applications. Chem. Rev. 2024, 124, 7533–7535. [Google Scholar] [CrossRef]
- Pan, F.; Peerless, B.; Dehnen, S. Bismuth-Based Metal Clusters horizontal line From Molecular Aesthetics to Contemporary Materials Science. Acc. Chem. Res. 2023, 56, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Köhler, D.; Ruck, M. Room-Temperature Synthesis of Bismuth Clusters in Ionic Liquids and Crystal Growth of Bi5(AlCl4)3. Z. Anorg. Allg. Chem. 2009, 635, 297–300. [Google Scholar] [CrossRef]
- Lindsjö, A.F.M.; Kloo, L. Improvements of and Insights into the Isolation of Bismuth Polycations from Benzene Solution—Single-Crystal Structure Determinations of Bi8[GaCl4]2 and Bi5[GaCl4]3. Eur. J. Inorg. Chem. 2005, 2005, 670–675. [Google Scholar] [CrossRef]
- Krebs, B.; Hucke, M.; Brendel, C.J. Structure of the Octabismuth(2+) Cluster in Crystalline Bi8(AlCl4)2. Angew. Chem. Int. Ed. 1982, 21, 445–446. [Google Scholar] [CrossRef]
- Knies, M.; Ruck, M. Bismuth-rich bimetallic clusters (CuBi8)3+ and [MBi10]4+ (M = Pd, Pt) from ionothermal synthesis. Z. Naturforsch. B, 2022; 77, 191–196. [Google Scholar]
- Groh, M.F.; Wolff, A.; Wahl, B.; Rasche, B.; Gebauer, P.; Ruck, M. Pentagonal Bismuth Antiprisms with Endohedral Palladium or Platinum Atoms by Low-Temperature Syntheses. Z. Anorg. Allg. Chem. 2016, 643, 69–80. [Google Scholar] [CrossRef]
- Groh, M.F.; Müller, U.; Isaeva, A.; Ruck, M. The Intermetalloid Clusters [Ni2Bi12]4+ and [Rh2Bi12]4+– Ionothermal Synthesis, Crystal Structures, and Chemical Bonding. Z. Anorg. Allg. Chem. 2018, 645, 161–169. [Google Scholar] [CrossRef]
- Groh, M.F.; Isaeva, A.; Ruck, M. [Ru2Bi14Br4](AlCl4)4 by mobilization and reorganization of complex clusters in ionic liquids. Chem. Eur. J. 2012, 18, 10886–10891. [Google Scholar] [CrossRef]
- Wahl, B.; Erbe, M.; Gerisch, A.; Kloo, L.; Ruck, M. Nobel-Metal Centered Polycations [Au@Bi10]5+ or [Pd@Bi10]4+ Embedded in Halogenido-Bismuthate(III)-Stannate(II) Frameworks. Z. Anorg. Allg. Chem. 2009, 635, 743–752. [Google Scholar] [CrossRef]
- Knies, M.; Kaiser, M.; Lê Anh, M.; Efimova, A.; Doert, T.; Ruck, M. Low-Temperature Ordering in the Cluster Compound (Bi8)Tl[AlCl4]3. Inorganics 2019, 7, 45. [Google Scholar] [CrossRef]
- Ahmed, E.; Ahrens, E.; Heise, M.; Ruck, M. A Facile Route for the Synthesis of Polycationic Tellurium Cluster Compounds: Synthesis in Ionic Liquid Media and Characterization by Single-Crystal X-Ray Crystallography and Magnetic Susceptibility. Z. Anorg. Allg. Chem. 2010, 636, 2602. [Google Scholar] [CrossRef]
- Ahmed, E.; Breternitz, J.; Groh, M.F.; Isaeva, A.; Ruck, M. [Sb7Se8Br2]3+ and [Sb13Se16Br2]5+—Double and Quadruple Spiro Cubanes from Ionic Liquids. Eur. J. Inorg. Chem. 2014, 19, 3037–3042. [Google Scholar] [CrossRef]
- Müller, U.; Isaeva, A.; Richter, J.; Knies, M.; Ruck, M. Polyhedral Bismuth Polycations Coordinating Gold(I) with Varied Hapticity in a Homoleptic Heavy-Metal Cluster. Eur. J. Inorg. Chem. 2016, 2016, 3580–3584. [Google Scholar] [CrossRef]
- Dubenskyy, V.; Ruck, M. Das Subchlorid Bi16PdCl22: [Pd@Bi10]4+-Polykationen in einem Raumnetzwerk aus Chlorobismutat(III)-Anionen. Z. Anorg. Allg. Chem. 2004, 630, 2458–2462. [Google Scholar] [CrossRef]
- Ruck, M.; Dubenskyy, V.; Sohnel, T. Structure and bonding of Pd@[Bi10]4+ in the subbromide Bi14PdBr16. Angew. Chem. Int. Ed. 2003, 42, 2978–2982. [Google Scholar] [CrossRef]
- Wilson, R.J.; Lichtenberger, N.; Weinert, B.; Dehnen, S. Intermetalloid and Heterometallic Clusters Combining p-Block (Semi)Metals with d- or f-Block Metals. Chem. Rev. 2019, 119, 8506–8554. [Google Scholar] [CrossRef]
- Wahl, B.; Ruck, M. Die molekularen Cluster [Bi10Au2](EBi3X9)2 (E = As, Bi; X = Cl, Br)—Synthese, Kristallstrukturen, Drillingsbildung und chemische Bindung. Z. Anorg. Allg. Chem. 2008, 634, 2267–2275. [Google Scholar] [CrossRef]
- King, R.B.; Silaghi-Dumitrescu, I.; Uţǎ, M.M. Beyond the Wade−Mingos Rules in Bare 10- and 12-Vertex Germanium Clusters: Transition States for Symmetry Breaking Processes. J. Chem. Theory Comput. 2008, 4, 209–215. [Google Scholar] [CrossRef]
- Michael, D.; Mingos, P. Polyhedral skeletal electron pair approach, Acc. Chem. Res. 1984, 17, 311–319. [Google Scholar] [CrossRef]
- Baranets, S.; Bobev, S. Ca14AlBi11—A new Zintl phase from earth-abundant elements with a great potential for thermoelectric energy conversion. Mater. Today Adv. 2020, 7, 100094. [Google Scholar] [CrossRef]
- Janzen, R.; Baranets, S.; Bobev, S. Synthesis and structural characterization of the new Zintl phases Eu10Mn6Bi12 and Yb10Zn6Sb12. Dalton Trans. 2022, 51, 13470–13478. [Google Scholar] [CrossRef]
- Mednikov, E.G.; Eremenko, N.K.; Mikhailov, V.A.; Gubin, S.P.; Slovokhotov, Y.L.; Struchkov, Y.T. New palladium cluster compounds. X-Ray crystal structure of [Pd10(CO)12(O)6. J. Chem. Soc. Chem. Commun. 1981; 989–990. [Google Scholar] [CrossRef]
- Baranets, S.; Ovchinnikov, A.; Dmitrenko, O.; Bobev, S. Structural Uniqueness of the [Nb(As5)2]5- Cluster in the Zintl Phase Cs5NbAs10. J. Phys. Chem. A 2021, 125, 4323–4333. [Google Scholar] [CrossRef] [PubMed]
- Stroganova, E.A.; Troyanov, S.I.; Morozov, I.V.; Kuznetsov, A.N. Bismuth Polycations Revisited: Alternative Synthesis and Electronic Structure of Bi6Br7, and Bonding in Main-Group Polyatomic Ions from a Direct Space Perspective. Crystals 2020, 10, 940. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SAINT 2014, Bruker AXS Inc.: Madison, Wisconsin, USA, 2014.
- Bruker AXS Inc. SADABS 2014, Bruker AXS Inc.: Madison, Wisconsin, USA, 2014.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Gelato, L.M.; Parthé, E. STRUCTURE TIDY—A Computer Program to Standardize Crystal Structure Data. J. Appl. Crystallogr. 1987, 20, 139–143. [Google Scholar] [CrossRef]
- Palmer, D.C. CrystalMaker; CrystalMaker Software Ltd.: Begbroke, Oxfordshire, UK, 2014. [Google Scholar]
- TURBOMOLE, V7.8. A Development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, TURBOMOLE GmbH 2024. Available online: http://www.turbomole.com (accessed on 15 November 2024).
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction, J. Chem. Phys. 1993, 98, 5648. [Google Scholar]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes, J. Chem. Phys. 2003, 119, 12129. [Google Scholar] [CrossRef]
- SGrimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets, J. Chem. Phys. 2015, 143, 054107. [Google Scholar]
- Hättig, C.; Schmitzand, J.G. Koßmann, Auxiliary basis sets for density-fitted correlated wavefunction calculations: Weighted core-valence and ECP basis sets for post-d elements. Phys. Chem. Chem. Phys. 2012, 14, 6549. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design an assessment of accuracy. Phys. Chem. Chem. Phys/ 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Weigend, F. Accurate coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. TmoleX—A graphical user interface for TURBOMOLE. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef]
- Jepsen, O.; Andersen, O.K. The Stuttgart TB-LMTO-ASA Program; Max-Planck-Institut für Festkörperforschung: Stuttgart, Germany, 1999. [Google Scholar]
- Barth, U.v.; Hedin, L. A Local Exchange-Correlation Potential for the Spin Polarized Case: I. J. Phys. C Solid State Phys. 1972, 5, 1629–1642. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).