trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Nitrate
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
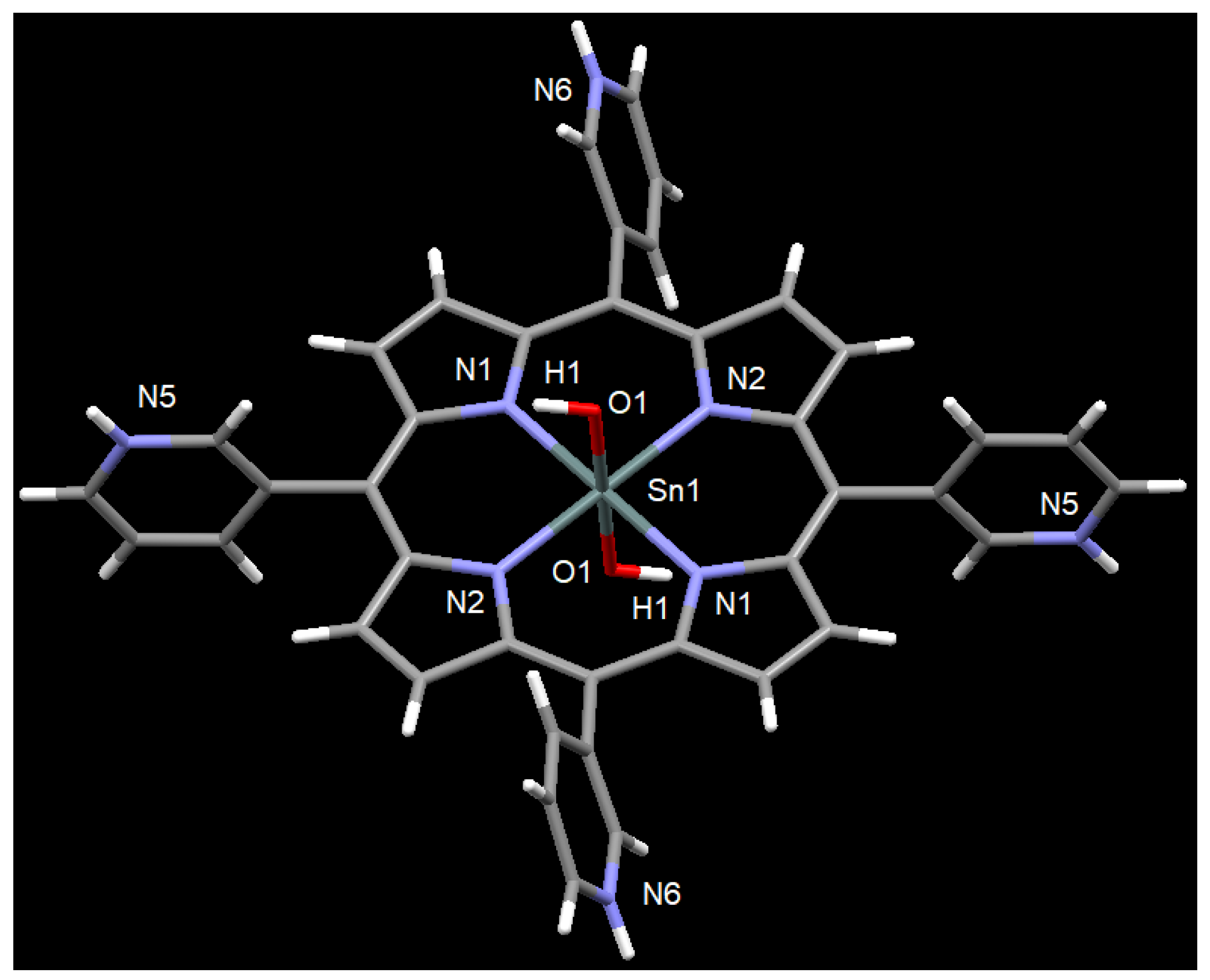
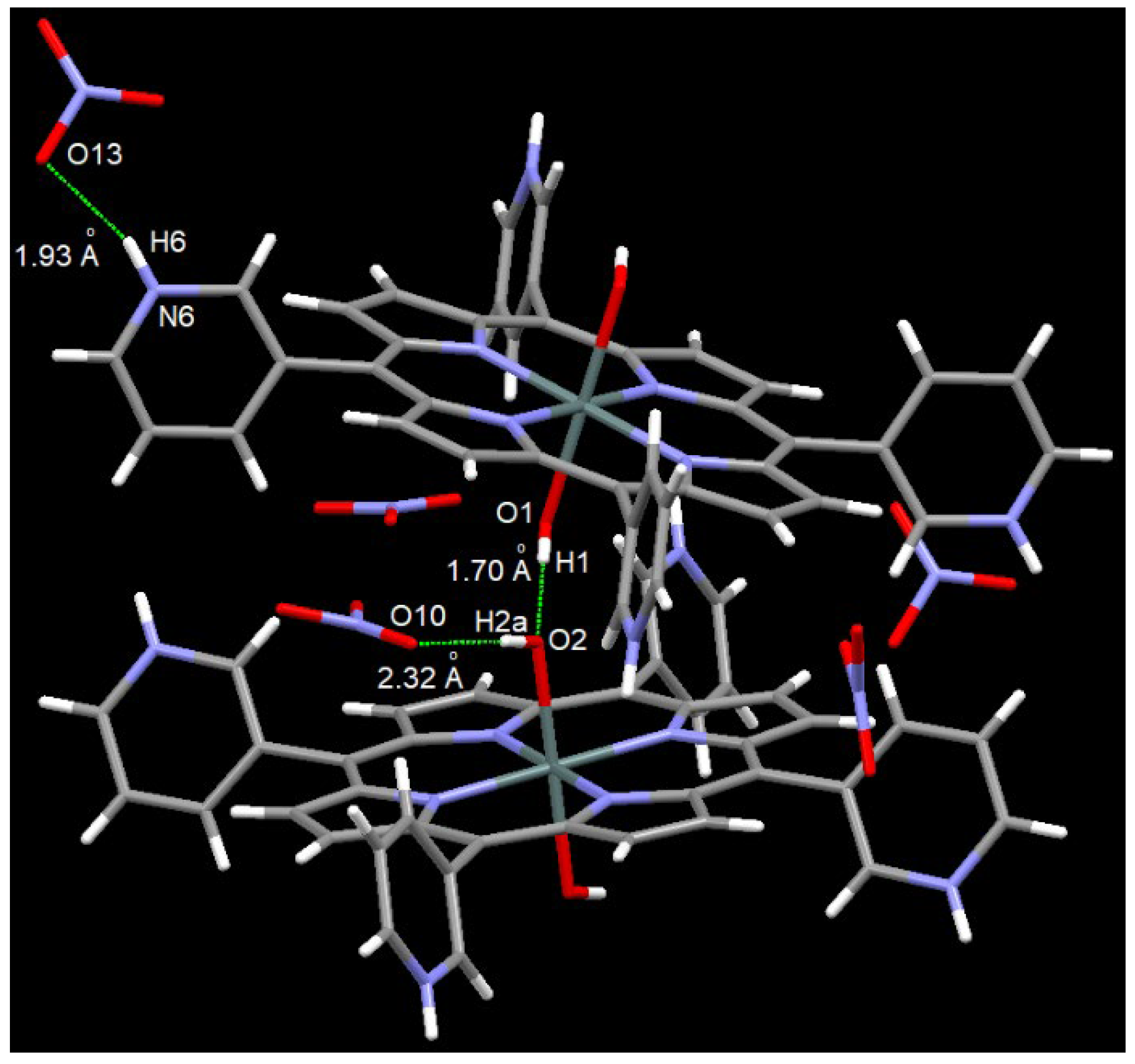
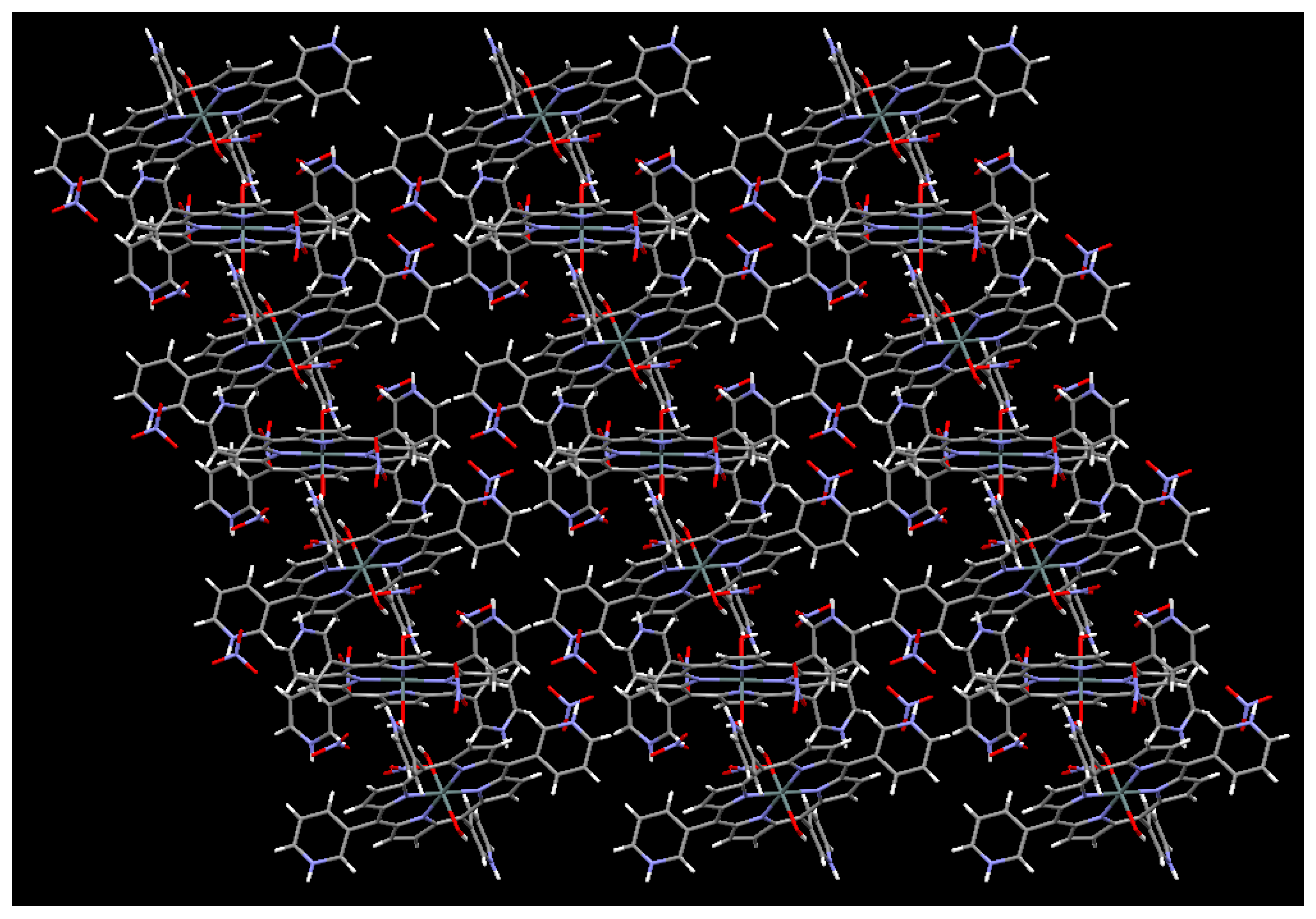
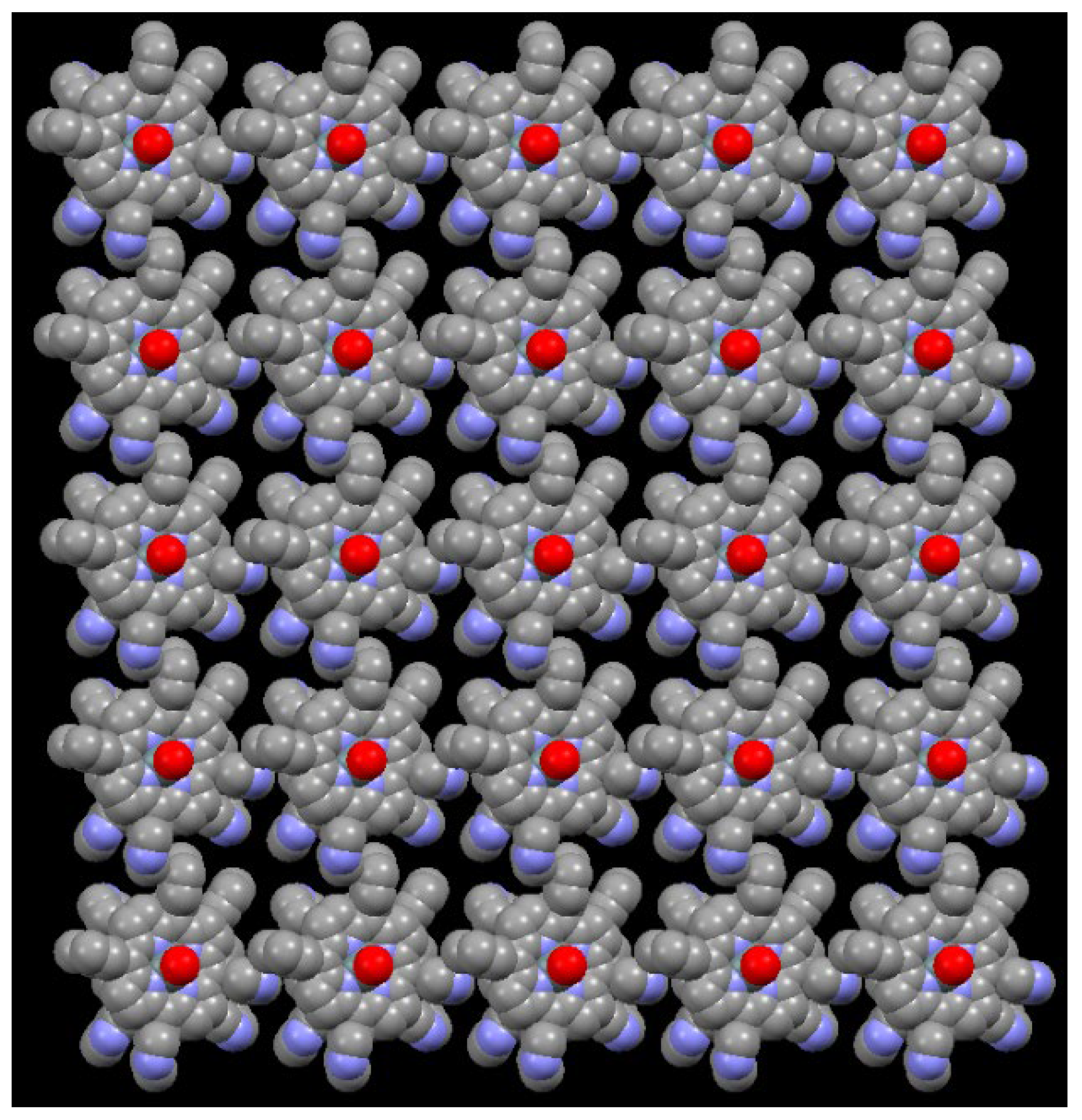
2.2. X-Ray Crystal Structure Determination
2.3. Spectroscopic Characterization
3. Materials and Methods
3.1. Preparation of trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Tetranitrate (1)
3.2. X-Ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrahams, B.F.; Hoskins, B.F.; Robson, R. A new type of infinite 3D polymeric network containing 4-connected, peripherally-linked metalloporphyrin building blocks. J. Am. Chem. Soc. 1991, 113, 3606–3607. [Google Scholar] [CrossRef]
- Khodov, I.; Alper, G.; Mamardashvili, G.; Mamardashvili, N. Hybrid multi-porphyrin supramolecular assemblies: Synthesis and structure elucidation by 2D DOSY NMR studies. J. Mol. Struct. 2015, 1099, 174–180. [Google Scholar] [CrossRef]
- Hu, J.S.; Guo, Y.G.; Liang, H.P.; Wan, L.J.; Jiang, L. Three-dimensional self-organization of supramolecular self-assembled porphyrin hollow hexagonal nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Ring, D.J.; Aragoni, M.C.; Champness, N.R.; Wilson, C. A coordination polymer supramolecular isomer formed from a single building block: An unexpected porphyrin ribbon constructed from zinc(tetra(4-pyridyl)porphyrin). CrystEngComm 2005, 7, 621–623. [Google Scholar] [CrossRef]
- Kellett, R.M.; Spiro, T.G. Cobalt(I) Porphyrin Catalysis of Hydrogen Production from Water. Inorg. Chem. 1985, 24, 2373–2377. [Google Scholar] [CrossRef]
- Liu, H.; Chen, S.; Zhang, Y.; Li, R.; Zhang, J.; Peng, T. An effective Z-scheme hybrid photocatalyst based on zinc porphyrin derivative and anatase titanium dioxide microsphere for carbon dioxide reduction. Mater. Today Sustain. 2022, 19, 100164. [Google Scholar] [CrossRef]
- Daphnomili, D.; Landrou, G.; Prakash Singh, S.; Thomas, A.; Yesudas, K.; Bhanuprakash, K.; Sharma, G.D.; Coutsolelos, A.G. Photophysical, electrochemical and photovoltaic properties of dye sensitized solar cells using a series of pyridyl functionalized porphyrin dyes. RSC Adv. 2012, 2, 12899–12908. [Google Scholar] [CrossRef]
- Ohmura, T.; Setoyama, N.; Mukae, Y.; Usuki, A.; Senda, S.; Matsumoto, T.; Tatsumi, K. Supramolecular porphyrin-based metal–organic frameworks: Cu(II) naphthoate–Cu(II) tetrapyridyl porphine structures exhibiting selective CO2/N2 separation. CrystEngComm 2017, 19, 5173–5177. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of Porphyrins in Antibacterial Photodynamic Therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Peng, R.; Offenhäusser, A.; Ermolenko, Y.; Mourzina, Y. Biomimetic sensor based on Mn(III) meso-tetra(N-methyl-4-pyridyl) porphyrin for non-enzymatic electrocatalytic determination of hydrogen peroxide and as an electrochemical transducer in oxidase biosensor for analysis of biological media. Sens. Actuators B Chem. 2020, 321, 128437. [Google Scholar] [CrossRef]
- Xie, M.-H.; Yang, X.-L.; Zou, C.; Wu, C.-D. A SnIV–Porphyrin-Based Metal-Organic Framework for the Selective Photo-Oxygenation of Phenol and Sulfides. Inorg. Chem. 2011, 50, 5318–5320. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanostructure of Ionic Sn(IV)porphyrin Complex Based on Multivalent Interactions for Photocatalytic Degradation of Water Contaminants. Molecules 2024, 29, 4200. [Google Scholar] [CrossRef] [PubMed]
- Langford, S.J.; Woodward, C.P. Supramolecular self-assembly of dihydroxy tin(IV) porphyrin stabilized helical water chains. CrystEngComm 2007, 9, 218–221. [Google Scholar] [CrossRef]
- Babu, B.; Soy, R.C.; Mack, J.; Nyokong, T. Non-aggregated lipophilic water-soluble tin porphyrins as photosensitizers for photodynamic therapy and photodynamic antimicrobial chemotherapy. New J. Chem. 2020, 44, 11006–11012. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Pigiaki, M.; Polychronaki, M.N.; Margaritopoulou, A.; Charalambidis, G.; Serpetzoglou, E.; Mitraki, A.; Loukakos, P.A.; Coutsolelos, A.G. Self-Assembly of Porphyrin Dipeptide Conjugates toward Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 7781–7791. [Google Scholar] [CrossRef]
- Likhonina, A.E.; Mamardashvili, G.M.; Khodov, I.A.; Mamardashvili, N.Z. Synthesis and Design of Hybrid Metalloporphyrin Polymers Based on Palladium (II) and Copper (II) Cations and Axial Complexes of Pyridyl-Substituted Sn(IV)Porphyrins with Octopamine. Polymers 2023, 15, 1055. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. (trans-Dihydroxo)Sn(IV)-[5,10,15,20-tetrakis(2-pyridyl)porphyrin]. Molbank 2023, 2023, M1669. [Google Scholar] [CrossRef]
- Kumar, A.A.; Giribabu, L.; Reddy, D.R.; Maiya, B.G. New molecular arrays based on a Tin(IV) porphyrin scaffold. Inorg. Chem. 2001, 40, 6757–6766. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Sn(IV) Porphyrin based axial-bonding type porphyrin triads containing heteroporphyrins as axial ligands. Inorg. Chem. 2010, 49, 2692–2700. [Google Scholar] [CrossRef]
- Amati, A.; Cavigli, P.; Demitri, N.; Natali, M.; Indelli, M.T.; Iengo, E. Sn(IV) multiporphyrin arrays as tunable photoactive systems. Inorg. Chem. 2019, 58, 4399–4411. [Google Scholar] [CrossRef]
- Thomas, A.; Ohsaki, Y.; Nakazato, R.; Kuttassery, F.; Mathew, S.; Remello, S.N.; Tachibana, H.; Inoue, H. Molecular Characteristics of Water-Insoluble Tin-Porphyrins for Designing the One-Photon-Induced Two-Electron Oxidation of Water in Artificial Photosynthesis. Molecules 2023, 28, 1882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; Wang, Z.; Busani, T.; Garcia, R.M.; Chen, Z.; Jiang, Y.; Song, Y.; Jacobsen, J.L.; Vu, T.T.; Schore, N.E.; et al. Donor-Acceptor Biomorphs from the Ionic Self-Assembly of Porphyrins. J. Am. Chem. Soc. 2010, 132, 8194–8201. [Google Scholar] [CrossRef]
- Martin, K.E.; Tian, Y.; Busani, T.; Medforth, C.J.; Franco, R.; van Swol, F.; Shelnutt, J.A. Charge Effects on the Structure and Composition of Porphyrin Binary Ionic Solids: ZnTPPS/SnTMePyP Nanomaterials. Chem. Mater. 2013, 25, 441–447. [Google Scholar] [CrossRef]
- Koposova, E.A.; Offenhäusser, A.; Ermolenko, Y.E.; Mourzina, Y.G. Photoresponsive Porphyrin Nanotubes of Meso-tetra(4-Sulfonatophenyl)Porphyrin and Sn(IV) meso-tetra(4-pyridyl)porphyrin. Front. Chem. 2019, 7, 351. [Google Scholar] [CrossRef]
- Jo, H.J.; Kim, S.H.; Kim, H.-J. Supramolecular Assembly of Tin(IV) Porphyrin Cations Stabilized by Ionic Hydrogen-Bonding Interactions. Bull. Korean Chem. Soc. 2015, 36, 2348–2351. [Google Scholar] [CrossRef]
- Kim, H.-J. Assembly of Sn(IV)-Porphyrin Cation Exhibiting Supramolecular Interactions of Anion·Anion and Anion·π Systems. Molbank 2022, 2022, M1454. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank 2023, 2023, M1723. [Google Scholar] [CrossRef]
- Jo, H.J.; Jung, S.H.; Kim, H.-J. Synthesis and Hydrogen-Bonded Supramolecular Assembly of Trans-Dihydroxotin (IV) Tetrapyridylporphyrin Complexes. Bull. Korean Chem. Soc. 2004, 25, 1869–1873. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Bruker. SHELXTL (Ver. 6.10): Program for Solution and Refinement of Crystal Structures; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Nitrate. Molbank 2025, 2025, M2014. https://doi.org/10.3390/M2014
Shee NK, Kim H-J. trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Nitrate. Molbank. 2025; 2025(2):M2014. https://doi.org/10.3390/M2014
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2025. "trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Nitrate" Molbank 2025, no. 2: M2014. https://doi.org/10.3390/M2014
APA StyleShee, N. K., & Kim, H.-J. (2025). trans-Dihydroxo[5,10,15,20-tetrakis(3-pyridinium)porphyrinato]tin(IV) Nitrate. Molbank, 2025(2), M2014. https://doi.org/10.3390/M2014





