2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one
Abstract
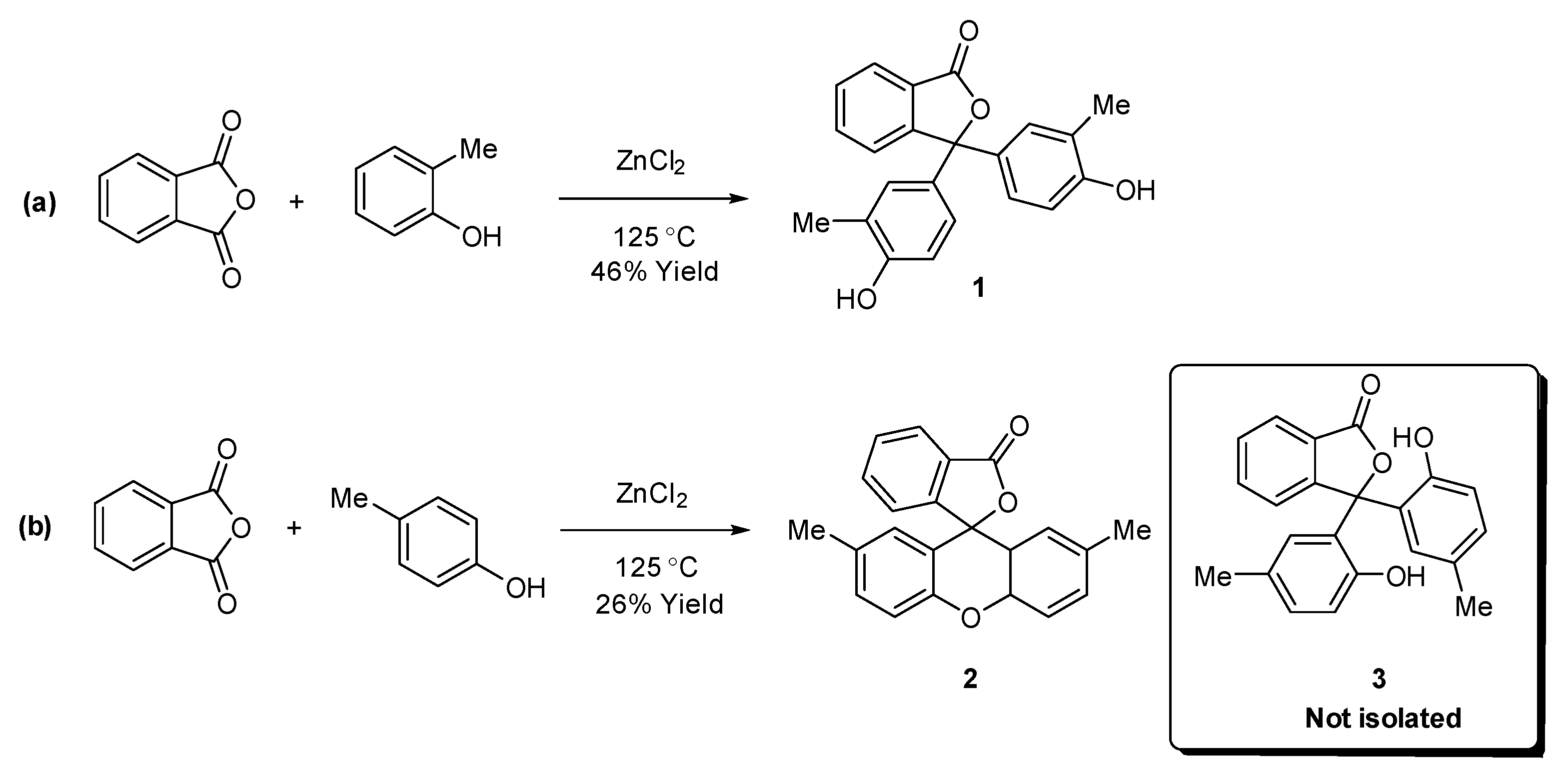
1. Introduction
2. Results
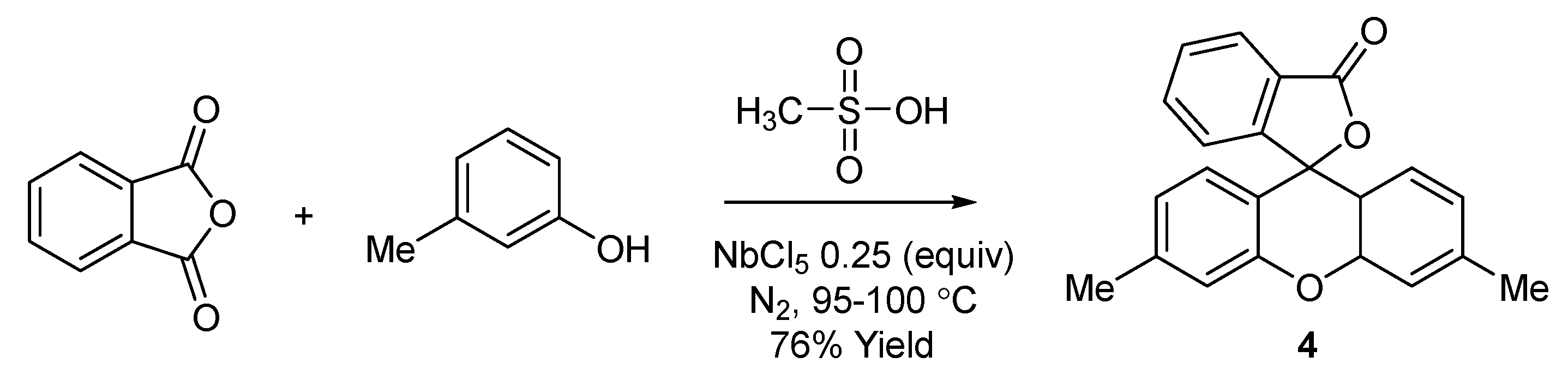
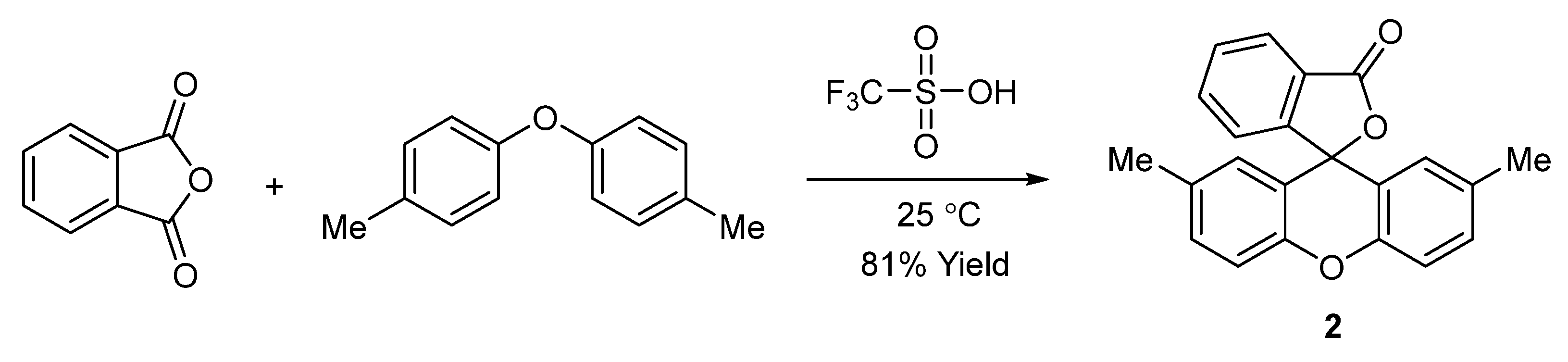
2.1. Synthesis and Spectroscopy
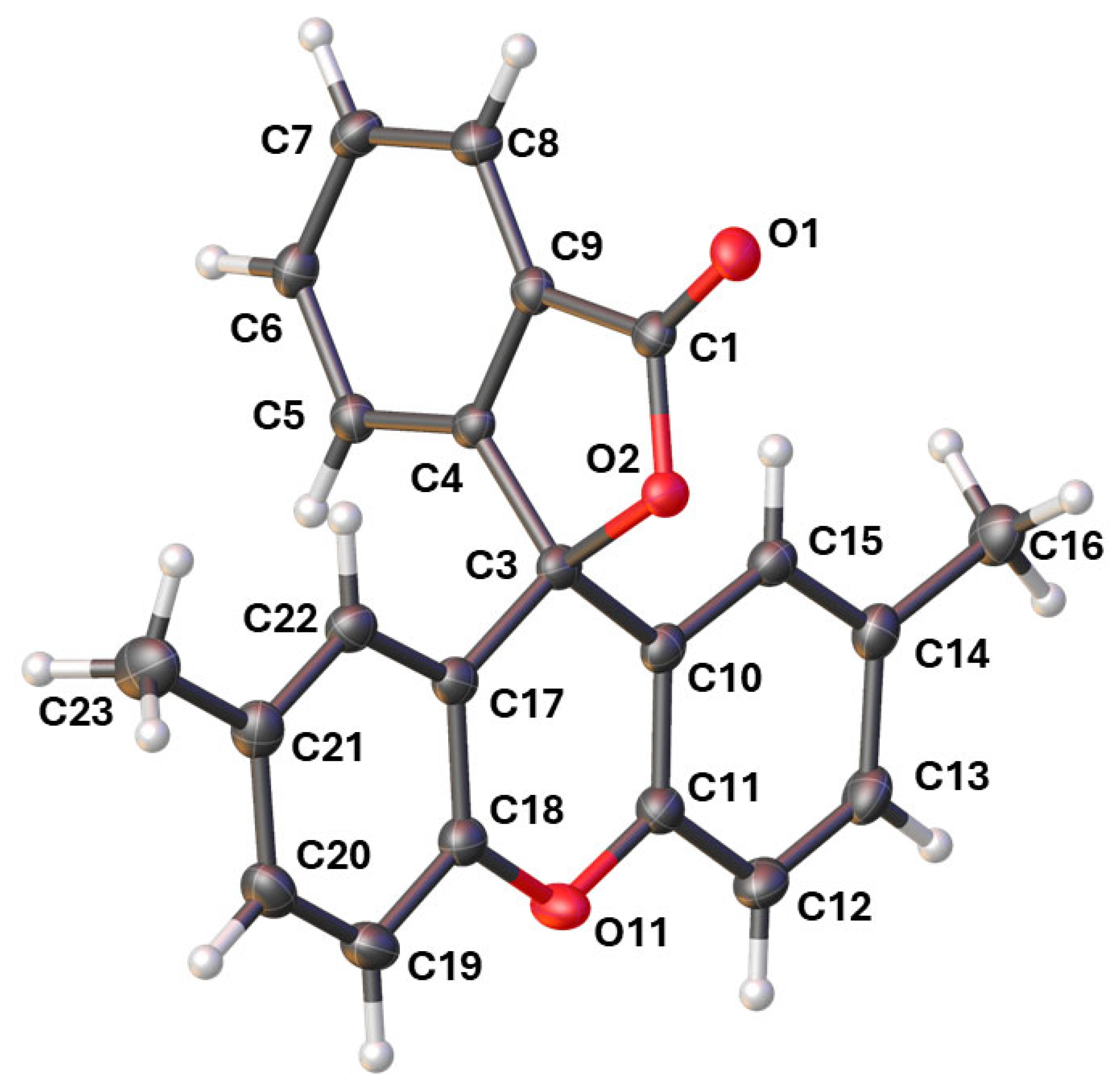
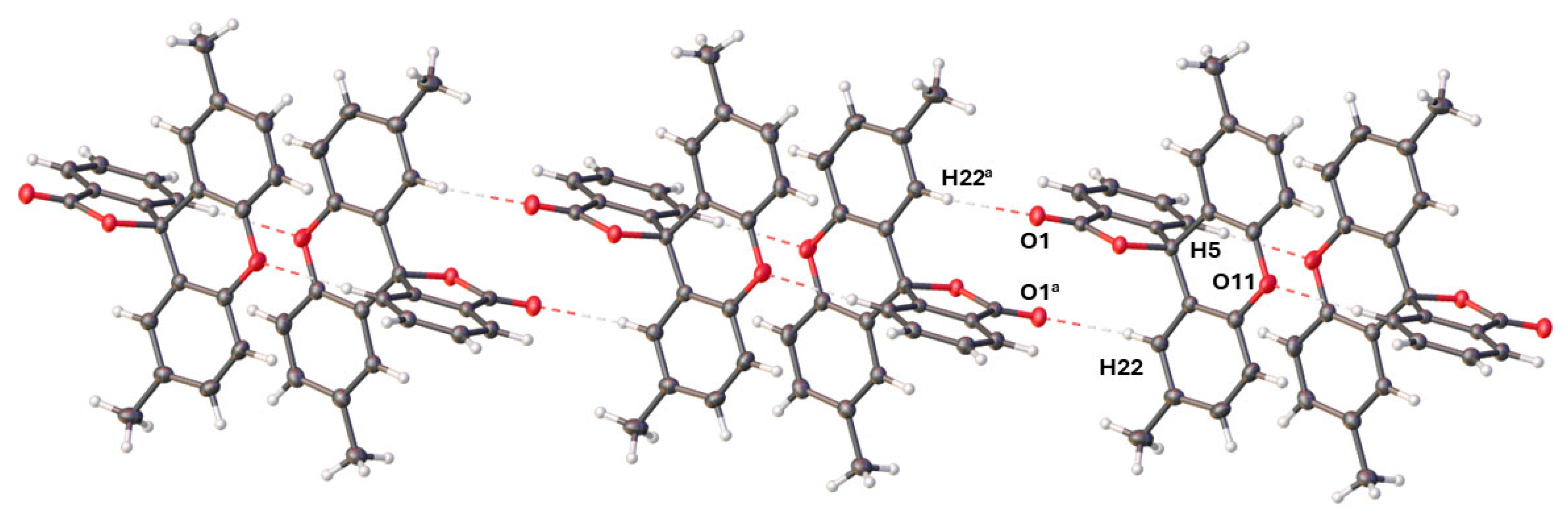
2.2. The Crystal Structure of 2
3. Experimental Section
- 2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one
- X-ray Structure Determination of 2
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabnis, R.W. A facile synthesis of phthalein dyes. Tetrahedron Lett. 2009, 50, 6261–6263. [Google Scholar] [CrossRef]
- Sabnis, R.W. Developments in the chemistry and applications of phthalein dyes. Part 1: Industrial applications. Coloration Technol. 2018, 134, 187–205. [Google Scholar] [CrossRef]
- Copisarow, M. XXVI—Phthaleins and Fluorans. J. Chem. Soc. Trans. 1920, 117, 209–218. [Google Scholar] [CrossRef]
- Bentley, W.H.; Gardner, H.D.; Weizmann, C. CLV-Researches on anthraquinones and phthaleins. J. Chem. Soc. Trans. 1907, 91, 1626–1640. [Google Scholar] [CrossRef]
- Moreno, V.F.; dos Santos, G.C.; da Costa, G.M.G.; Gomes, M.H.A.; da Silva-Filho, L.C. NbCl5 Promoted the efficient synthesis of phthalein derivatives: Optical characterization and solvatochromic effect. J. Heterocycl. Chem. 2019, 56, 2811–2821. [Google Scholar] [CrossRef]
- da Silva, B.H.S.T.; Bregadiollim, B.A.; Graeff, C.F.d.O.; da Silva-Filho, L.C. NbCl5 Promoted synthesis of fluorescein dye derivatives: Spectroscopic characterization and their application in dye-sensitised solar cels. ChemPlusChem 2017, 82, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Genaev, A.M.; Salnikov, G.E.; Koltunov, K.Y. Triflic acid-mediated condensation of phthalimide with diaryl ethers as a route to spiro-isoindolinones: Mechanistic insights and related reactions. J. Org. Chem. 2024, 89, 15931–15940. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Gallagher, B.S. The infrared spectra of steroid lactones. J. Am. Chem. Soc. 1959, 81, 5242–5251. [Google Scholar] [CrossRef]
- Hewitt, J.T.; Tervet, J.N. LXIX—Oxonium Salts of Fluoran and its Derivatives. J. Chem. Soc. Trans. 1902, 81, 663–666. [Google Scholar] [CrossRef]
- CrysAlisPro; v1.171.42.94a & 109a; Rigaku Oxford Diffraction; Rigaku Corporation: Oxford, UK, 2024.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmers, B.A.; McKay, A.P.; Cordes, D.B.; Patterson, I.L.J.; Vladymyrova, N.; Smellie, I.A. 2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one. Molbank 2025, 2025, M2007. https://doi.org/10.3390/M2007
Chalmers BA, McKay AP, Cordes DB, Patterson ILJ, Vladymyrova N, Smellie IA. 2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one. Molbank. 2025; 2025(2):M2007. https://doi.org/10.3390/M2007
Chicago/Turabian StyleChalmers, Brian A., Aidan P. McKay, David B. Cordes, Iain L. J. Patterson, Nadiia Vladymyrova, and Iain A. Smellie. 2025. "2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one" Molbank 2025, no. 2: M2007. https://doi.org/10.3390/M2007
APA StyleChalmers, B. A., McKay, A. P., Cordes, D. B., Patterson, I. L. J., Vladymyrova, N., & Smellie, I. A. (2025). 2′,7′-Dimethyl-3H-spiro[isobenzofuran-1,9′-xanthen]-3-one. Molbank, 2025(2), M2007. https://doi.org/10.3390/M2007






