Abstract
The title compound, i.e., (Z)-4-(azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one (3), has previously been synthesized in a good yield (56%) using the Erlenmeyer–Plöchl procedure. The crystal structure of 3 is described herein along with the results of a Hirshfeld surface analysis. Crystals of compound 3 were obtained through the slow evaporation of a mixture of petroleum ether/dichloromethane = 1:1 (vol.) at room temperature. This compound has a monoclinic system: a P21/n space group at room temperature. Its crystal packing is driven by π-π interactions between neighboring molecules in the range of 3.60–3.71 Å and by hydrogen bonds (D-H···A = 2.58 Å).
1. Introduction
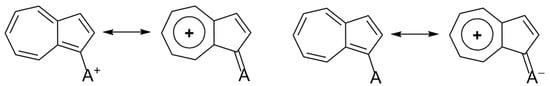
Naphthalene and azulene are two bicyclic isomeric systems corresponding to Huckel’s rule, with 4n + 2 = 10 π electrons. While the naphthalene, with two six-membered rings, has an alternate, benzenoid π electronic conjugation, azulene, with five- and seven-membered rings, possesses a non-alternating electronic structure [1]. One π electron is transferred from the seven-membered ring to the five-membered ring; therefore, azulene adopts a tropylium cation structure for the first ring and a cyclopentadienyl anion structure for the second (Scheme 1). The large dipole moment (µ = 1.08 D) and the blue color of azulene can be explained by this intramolecular charge transfer (Scheme 1).
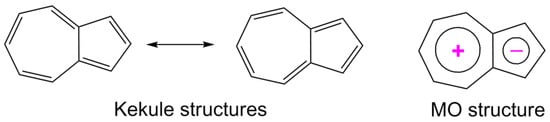
Scheme 1.
Resonance and MO structure of azulene.
The special structural and electronic properties of azulene compounds have generated interest in using them for technical purposes [2,3,4,5,6,7,8]. Thus, due to the polarizability of the azulene molecule, the azulen-1-yl group exhibits a strong electron donor characteristic (Scheme 2) [9], being a building block for obtaining push–pull systems with advanced optoelectronic properties [10,11,12].
Scheme 2.
The electron-donating effect of the azulen-1-yl moiety.
When a C=C bond is intercalated between the azulen-1-yl moiety and an electron acceptor [3], the obtained push–pull systems exhibit high hyperpolarizability, being used as possible candidates for NLO systems [13,14,15,16].
Several 1-vinylazulenes with heterocycles linked to azulene through a double bond were synthesized by our research group [2,17,18], and the condensation of azulene-1-carbaldehydes with compounds containing activated methyl or methylene groups was preferred for this purpose. Among such compounds are also the azulen-1-ylmethylene oxazolones, which have chemosensing properties suitable for the detection of heavy metals in water [19]. The potential technical use of azulene-1-ylmethylene 1 and 2 (Figure 1) [20,21] encouraged us to study the synthesis, characterization, and properties as chemically modified electrodes of 4-(azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one, 3 [22].
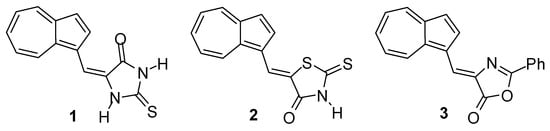
Figure 1.
Chemical structure of heterocyclic azulen-1-ylmethylenes.
Compound 3 was obtained by Cristea et al. [22] according to the Erlenmeyer–Plöchl procedure: azulene-1-carbaldehyde, 4, was condensed with hippuric acid, 5, a compound with an active ethylene unit, at reflux in an inert atmosphere (Scheme 3). Acetic anhydride was used as a solvent and dehydrating agent in the presence of AcONa, serving as a catalyst. The product, 3, was obtained in a good yield (56%) and completely characterized [22].
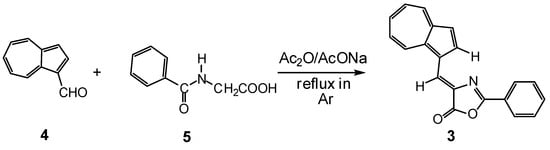
Scheme 3.
Synthesis of 4-(azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one 3.
This study presents the supramolecular features of (Z)-4-(azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one (Figure 1) described via single-crystal X-ray diffraction and Hirshfeld analysis. Through the functionalization of compound 3 (Figure 1) at the azulene or oxazolone core, a new class of 1-vinylazulene derivatives could emerge, with new, interesting properties suitable for application as, for example, alkyl substituted analogues [23,24].
2. Results and Discussion
Compound 3 was obtained in a good yield (56%) using to the Erlenmeyer–Plöchl procedure, which has been described previously [22]. This synthesis method consists of the condensation of azulene-1-carbaldehyde with hipuric acid under reflux conditions in an inert atmosphere in the presence of acetic anhydride and AcONa as a catalyst.
Crystals of 3 suitable for X-ray diffraction on a single crystal were obtained via the slow evaporation (at room temperature) of a mixture of petroleum ether/dichloromethane =1:1 (vol.) after seven days.
The title compound, C20H13NO2, crystallizes in the monoclinic system, with a P21/n space group (Figure 2).
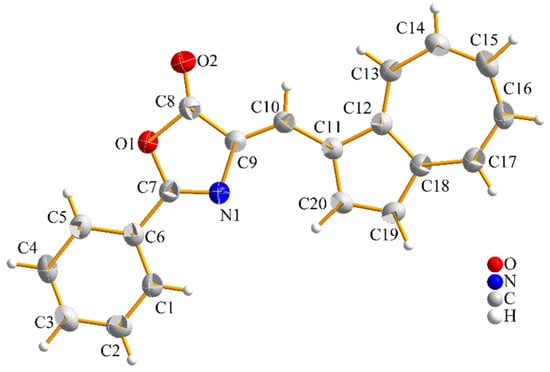
Figure 2.
Molecular structure and atom numbering scheme of 3, with anisotropic displacement ellipsoids drawn at 50% probability level. Bond distances (Å): O1–C7 = 1.3820(18), O1–C8 = 1.401(2), O2–C8 = 1.201(2), N1–C9 = 1.402(2), N1–C7 = 1.285(2), C12–C11 = 1.424(2), C12–C18 = 1.470(2), C12–C13 = 1.391(2), C6–C7 = 1.460(2), C6–C1 = 1.393(2), C6–C7 = 1.387(2), C11–C10 = 1.417(2), C11–C20 = 1.419(2), C9–C10 = 1.356(2), C9–C8 = 1.455(2), C18–C19 = 1.409(2), C18–C17 = 1.384(2), C13–C14 = 1.385(2), C20–C19 = 1.368(2), C1–C2 = 1.377(2), C5–C4 = 1.382(2), C17–C16 = 1.392(2), C14–C15 = 1.383(3), C2–C3 = 1.380(3), C3–C4 = 1.379(3), and C16–C15 = 1.378(3).
The packing diagram of 3 reveals π–π stacking between neighboring molecules with respect to azulene and benzene aromatic rings in the range of 3.60–3.71Å (3.88 Å considering centroid–centroid distance) and intermolecular hydrogen bonds, with D–H···A = 2.58 Å (D···A = 3.44 Å) (see Figure 3).
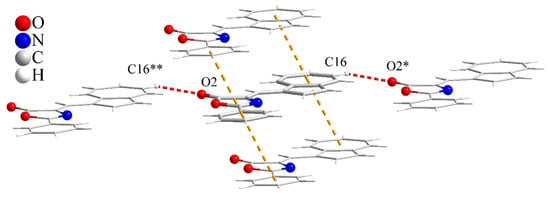
Figure 3.
View of the hydrogen and π-π interactions within the crystal structure of 3. C16-H···O2* = 2.58 Å (C16···O2* = 3.44 Å), C16-H-O2* = 155.4°. Symmetry operations: * = 1 + x, −1 + y, z; ** = −1 + x, 1 + y, z.
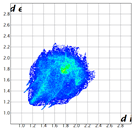
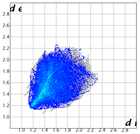
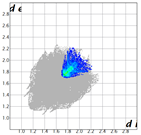
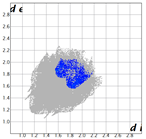
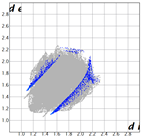
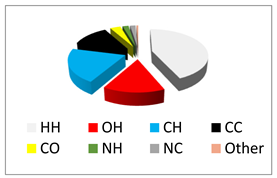
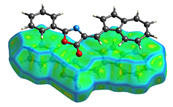
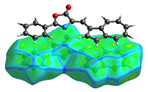
Hirshfeld analysis and energy frameworks were generated using Crystal Explorer 17 [25]. Fingerprint plots (Table 1) generated from the Hirshfeld surface show a compact structure dominated by H···H and C···H contacts in terms of percentage but driven by O···H and π···π stacking (C···C contacts) in terms of strength.

Table 1.
Fingerprint plots of and interaction percentages in the crystal structure of compound 3.
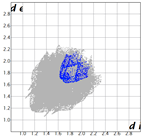
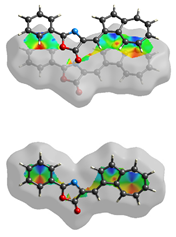
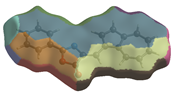
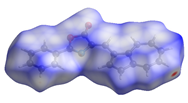
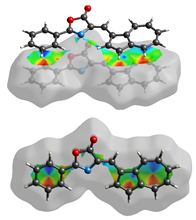
The Hirshfeld surface (Table 2) in dNorm presents two red spots for the most important H-bond between the H16 from azulene and C=O in the oxazolone ring. The Shapeindex mode of the Hirshfeld surface and Curvedness highlight the complementarity zones characteristic of π-stacking on both sides of the molecule. The fragment patch implies the presence of 15 neighboring molecules responsible for the entire interaction landscape and mainly for H···H and C···H contacts.

Table 2.
Hirshfeld surface of compound 3 generated for each mode.
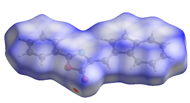
The supramolecular network is constructed on ribbons formed through O···H contacts, which are assembled in 3D via π-stacking. These 3D structures interact with the crystalline network through dispersive forces (Figure 4).
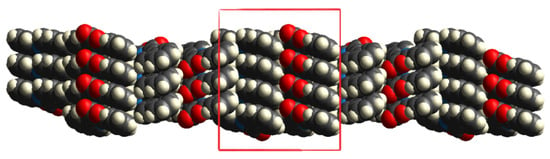
Figure 4.
3D molecular unit formed by O···H and π···π (red square) interactions and bonded to other similar structures.
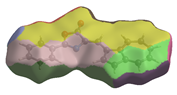
Energy frameworks were generated at the HF/3-21 level. They depict the most relevant interactions within the crystal. The dominant force is the potential component generated by the π···π stacking (Figure 5).
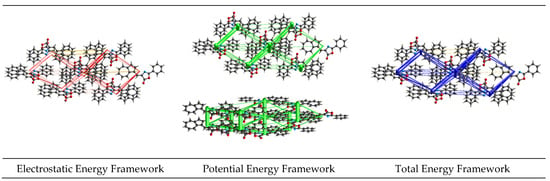
Figure 5.
Energy frameworks highlighting the most important interactions within the supramolecular structure of compound 3.
Compared to the substituted structures (CCDC: 2204908 and 2367271) [23,24], the parent structure shows fewer H···H contacts but more O···H and C···C contacts, which is expected. C···H contacts are similar. All three molecules present H-bonds that involve C=O groups from the oxazolonic rings. π-stacking seems to be a significant driving force for the title parent compound 3, with the π···π dimer energy (Figure 5) presenting rather lower values (−46.6 kJ/mol). The π-stacking forces act between similar moieties, with phenyl-phenyl, azulene–azulene, and the oxazolone ring not being involved in the same way as the alkyl-substituted analogues with an antiparallel or offset molecular configuration [23,24].
3. Materials and Methods
Single-crystal X-ray diffraction data were collected using XtaLAB Synergy, Dualflex, and HyPix diffractometers using Cu Kα radiation. Unit cell determination and data integration were carried out using the CrysAlisPro package from Oxford Diffraction [26]. The multi-scan correction for absorption was applied. The structure was solved using the SHELXT program via the intrinsic phasing method and refined via the full-matrix least-squares method on F2 with SHELXL [27,28]. Olex2 was used as an interface for the SHELX programs [29]. An anisotropic model was used for the refinement of non-hydrogen atoms. Hydrogen atoms were added in idealized positions and refined using a riding model. Selected crystallographic data and structure refinement details are provided in Table 3 and the corresponding CIF files. The crystallographic Supplementary Data can be obtained free of charge via accessing the following link: http://www.ccdc.cam.ac.uk, accessed on 21 March 2025 (they can also be obtained from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; (Tel: (+44) 1223 336 408; Fax: (+44) 1223-336-033; or deposit@ccdc.cam.ac.uk).

Table 3.
Crystallographic data, details of data collection, and structure refinement parameters for compound 3.
4. Conclusions
The parent compound of a new azulenemethylene oxazolone class, (Z)-4-(azulen-1-ylmethylene)-2-phenyloxazol-5(4H)-one, 3, was investigated regarding its solid-state properties through single-crystal X-ray diffraction and Hirshfeld characterization of the most important supramolecular features. The crystalline network is driven by H-bonding and π-stacking and stabilized by other dispersive forces. Compared to other alkyl-substituted analogues from its class, the parent compound displays a more compact structure and less hydrophobic interactions. Further functionalization of the parent compound and the generation of a library for this class of compounds are desirable due to their applicative potential.
Supplementary Materials
Figure S1. 1H-NMR spectrum of compound 3; Figure S2. 13C-NMR spectrum of compound 3.
Author Contributions
Conceptualization, M.C., and M.-M.P.; methodology, M.C., S.S., M.-M.P., and M.R.; software, S.S., M.-M.P., M.R., M.C., and A.C.R.; validation, M.C., S.S., M.-M.P., and M.R.; formal analysis, S.S., and M.R.; investigation, M.C., S.S., M.-M.P., and M.R.; resources, M.C., A.C.R., F.D., S.S., M.-M.P., and M.R.; data curation, M.-M.P., and M.R.; writing—original draft preparation, M.C., M.-M.P., A.C.R., and M.R.; writing—review and editing, M.C., M.-M.P., A.C.R., M.R., and F.D.; visualization, M.C., M.-M.P., A.C.R., M.R., S.S., and F.D.; supervision, M.C., M.-M.P., A.C.R., and F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
CCDC 2433119 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/structures (accessed on 21 March 2025).
Acknowledgments
M.C. would like to thank Cristian Enache for providing the solvents necessary for the chromatography of the title compound and Calin Deleanu and Alina Nicolescu for the NMR spectra.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zeller, K.-P. Methoden der Organischen Chemie; G. Thieme Verlag: Stuttgart, NY, USA, 1985; Volume V/2c, pp. 127–416. [Google Scholar]
- Razus, A.C.; Birzan, L. Synthesis of azulenic compounds with a homo- or hetero-atomic double bond at position. Arkivoc 2018, IV, 1–56. [Google Scholar] [CrossRef]
- Zadeh Ghazvini, E.H.; Tang, S.; Woodward, A.; Liu, W.T.; Bondar, M.V.; Belfield, K.D. Chromophoric materials derived from a natural azulene: Syntheses, halochromism and one-photon and two-photon microlithography. J. Mater. Chem. C 2015, 3, 8495–8503. [Google Scholar] [CrossRef]
- Koch, M.; Blacque, O.; Venkatesan, K. Impact of 2,6-connectivity in azulene: Optical properties and stimuli responsive behavior. J. Mater. Chem. C 2013, 1, 7400–7408. [Google Scholar] [CrossRef]
- Ito, S.; Morita, N. Creation of stabilized electrochromic materials by taking advantage of azulene skeletons. Eur. J. Org. Chem. 2009, 27, 4567–4579. [Google Scholar] [CrossRef]
- Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C.J.; Robb, M.J. Modulating the Properties of Azulene-Containing Polymers through Controlled Incorporation of Regioisomers. Adv. Func. Mater. 2014, 24, 7338–7347. [Google Scholar] [CrossRef]
- Wang, X.; Ng, J.K.-P.; Jia, P.; Lin, T.; Cho, C.M.; Xu, J.; Lu, X.; He, C. Synthesis, electronic, and emission spectroscopy, and electrochromic characterization of azulene–fluorene conjugated oligomers and polymers. Macromolecules 2009, 42, 5534–5544. [Google Scholar] [CrossRef]
- Amir, E.; Amir, R.J.; Campos, L.M.; Hawker, C.J. Stimuli-responsive azulene-based conjugated oligomers with polyaniline-like properties. J. Am. Chem. Soc. 2011, 133, 10046–10049. [Google Scholar] [CrossRef]
- Razus, A.C. Azulene Moiety as Electron Reservoir in Positive Charged Systems; Short Survey. Symmetry 2021, 13, 526. [Google Scholar] [CrossRef]
- Lopez-Alled, C.M.; Park, S.J.; Lee, D.J.; Murfin, L.C.; Kociok-Kohn, G.; Hann, J.L.; Wenk, J.; James, T.D.; Kim, H.M.; Lewis, S.E. Azulene-based fluorescent chemosensor for adenosine diphosphate. Chem. Commun. 2021, 57, 10608–10611. [Google Scholar] [CrossRef]
- Dong, J.-X.; Zhang, H.-L. Azulene-based organic functional molecules for optoelectronics. Chin. Chem. Lett. 2016, 27, 1097–1104. [Google Scholar] [CrossRef]
- Gao, H.; Ge, C.; Hou, B.; Xin, H.; Gao, X. Incorporation of 1,3-Free-2,6-Connected Azulene Units into the Back-bone of Conjugated Polymers: Improving Proton Responsiveness and Electrical Conductivity. ACS Macro Lett. 2019, 8, 1360–1364. [Google Scholar] [CrossRef]
- Herrmann, R.; Pedersen, B.; Wagner, G.; Youn, J.-H. Molecules with potential applications for non-linear optics: The combination of ferrocene and azulene. J. Organomet. Chem. 1998, 571, 261–266. [Google Scholar] [CrossRef]
- Iftime, G.; Lacroix, P.G.; Nakatani, K.; Razus, A.C. Push-pull azulene- based chromophores with nonlinear optical properties. Tetrahedron Lett. 1998, 39, 6853–6856. [Google Scholar] [CrossRef]
- Asato, A.E.; Liu, R.S.; Rao, V.P.; Cai, Y.M. Azulene-containing donor-acceptor compounds as second-order nonlinear chromophores. Tetrahedron Lett. 1996, 37, 419–422. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, P.; Ye, C.; Asato, A.E.; Liu, R.S. Theoretical investigation and molecular design of some azulene derivatives with large hyperpolarizabilities. J. Phys. Chem. A 1999, 103, 7076–7082. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Razus, A.C.; Buica, G.O.; Ungureanu, E.-M. Vinylazulenes chromophores: Synthesis and characterization. Dyes Pigm. 2016, 131, 246–255. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Arnold, G.-L.; Ungureanu, E.-M.; Razus, A.C. 1-Vinylazulenes-potential host molecules in ligands for metal ion detectors. Tetrahedron 2016, 72, 2316–2326. [Google Scholar] [CrossRef]
- Brotea, A.-G.; Matica, O.-T.; Musina (Borsaru), C.; Pandele, A.M.; Trusca, R.; Ungureanu, E.-M. Chemically Modified Electrodes Based on 4-((5-Isopropyl-3, 8-dimethylazulen-1-yl) methylene)-2-phenyloxazol-5 (4H)-one. Symmetry 2024, 16, 245. [Google Scholar] [CrossRef]
- Stefaniu, A.; Pop, M.-D.; Arnold, G.-L.; Birzan, L.; Pintilie, L.; Diacu, E.; Ungureanu, E.-M. DFT calculations and electrochemical studies on azulene ligands for heavy metal ions detection using chemically modified electrodes. J. Electrochem. Sci. Eng. 2018, 8, 73–85. [Google Scholar] [CrossRef]
- Ungureanu, E.-M.; Popescu (Apostoiu), M.; Tatu (Arnold), G.-L.; Birzan, L.; Isopescu, R.; Stanciu, G.; Buica, G.-O. Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones. Symmetry 2021, 13, 588. [Google Scholar] [CrossRef]
- Cristea, M.; Bîrzan, L.; Dumitrascu, F.; Enache, C.; Tecuceanu, V.; Hanganu, A.; Drăghici, C.; Deleanu, C.; Nicolescu, A.; Maganu, M.; et al. 1-Vinylazulenes with Oxazolonic Ring-Potential Ligands for Metal Ion Detectors; Synthesis and Products Properties. Symmetry 2021, 13, 1209. [Google Scholar] [CrossRef]
- Răducă, M.; Raţ, C.I.; Cristea, M. Crystal structure and Hirshfeld surface analysis of (Z)-2-Phenyl-4-((4,6,8-trimethylazulen-1-yl)methylene)oxazol-5(4H)-one. Rev. Roum. Chim. 2022, 67, 591–596. [Google Scholar] [CrossRef]
- Răducă, M.; Popa, M.M.; Cristea, M.; Razus, A.C.; Dumitrascu, F. Crystal structure and Hirshfeld surface analysis of (Z)-4-((5-isopropyl-3,8-dimethylazulen-1-yl)methylene)-2-phenyloxazol-5(4H)-one. Rev. Roum. Chim. 2025. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysAlis Pro Software System; Rigaku Corporation: Oxford, UK, 2015; CrysAlis Pro v. 1.171.38.46. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).