(E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one
Abstract
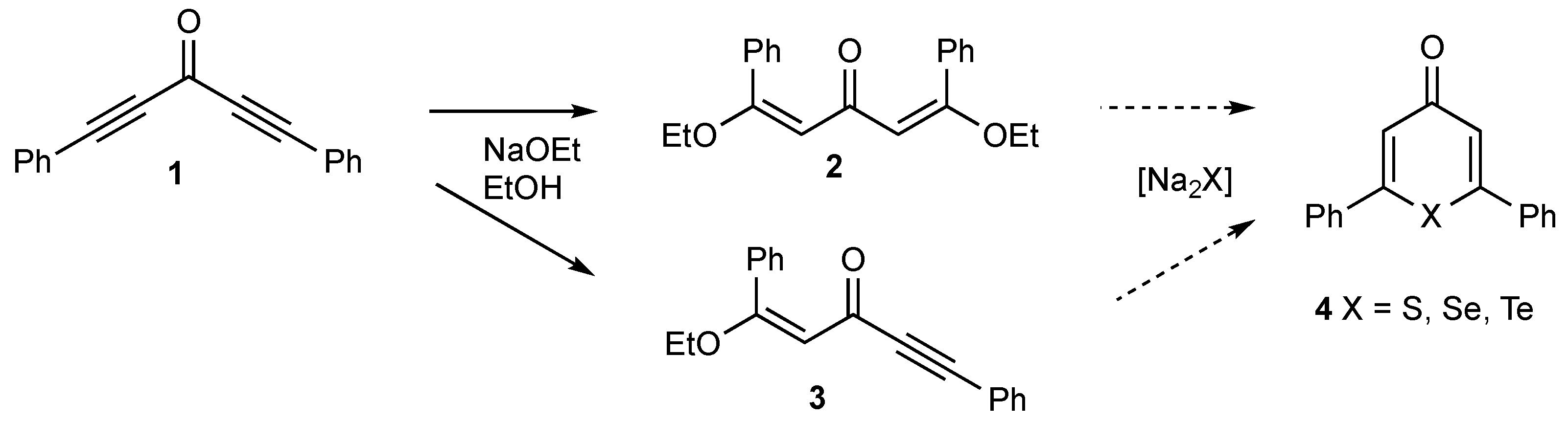
1. Introduction
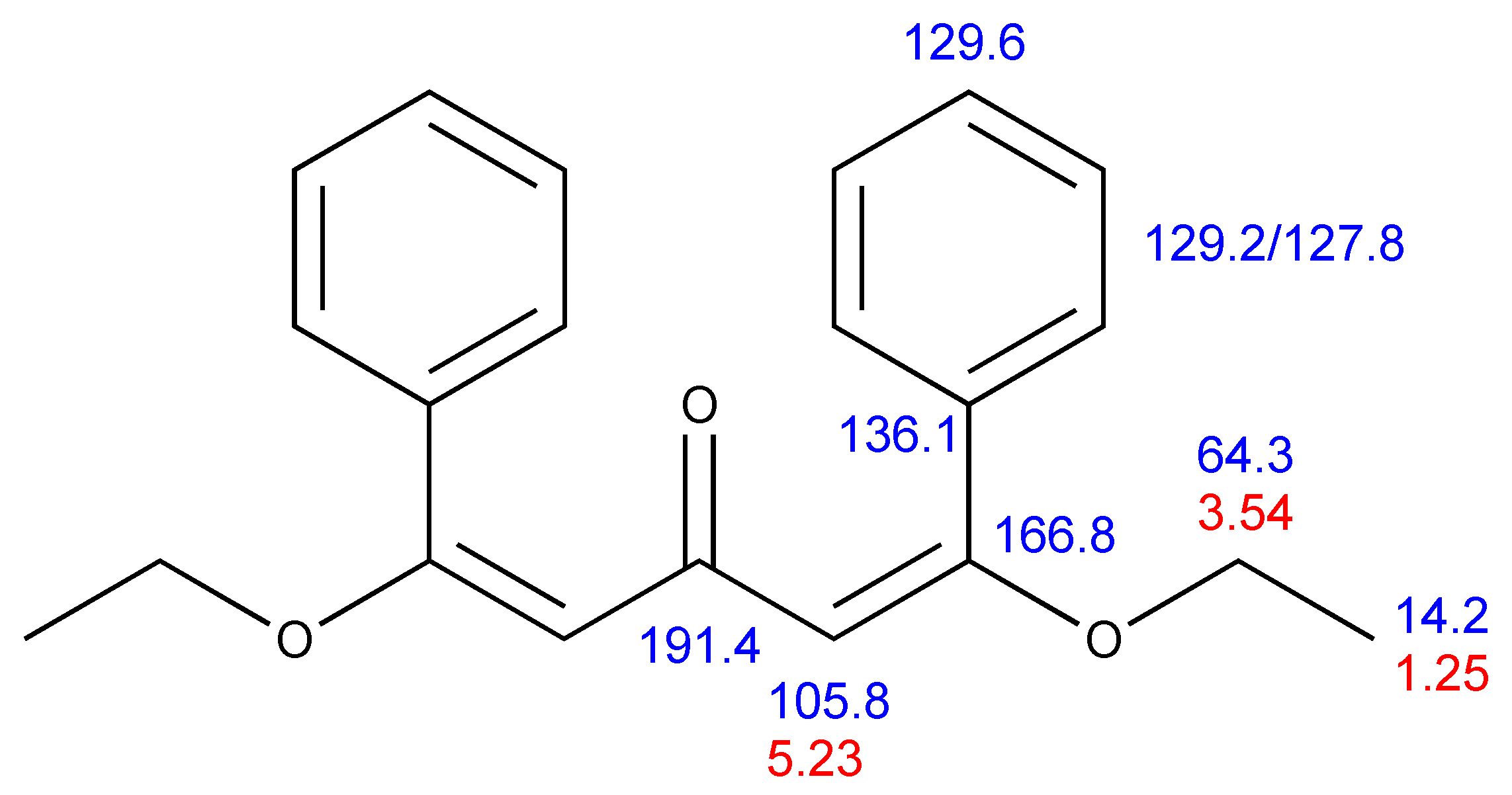
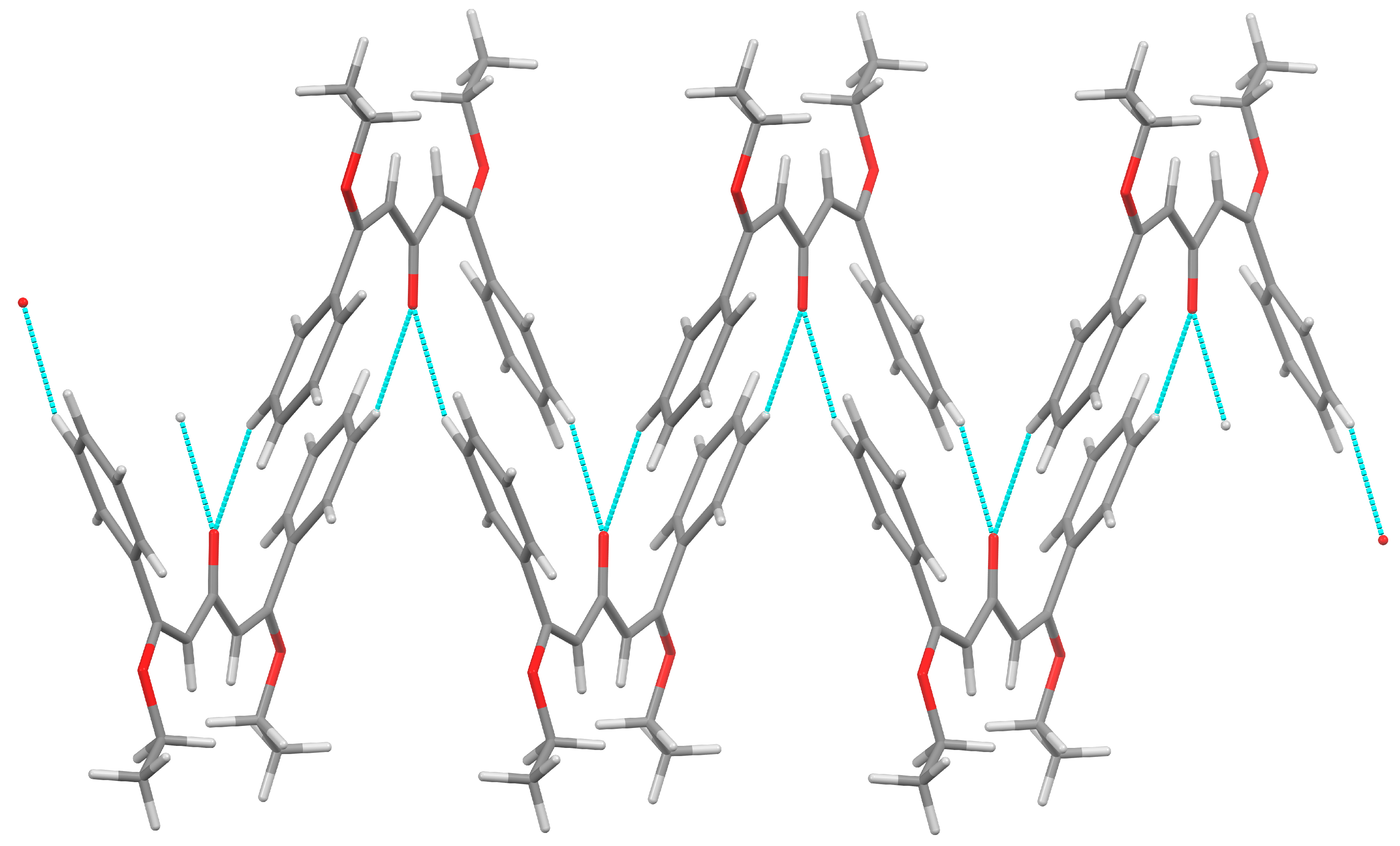
2. Results
3. Experimental
3.1. General Experimental Details
3.2. Synthesis of (E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one 2
3.3. X-Ray Structure Determination of 2
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vereshchagin, L.I.; Sushkova, N.V.; Vologdina, L.P. Unsaturated carbonyl-containing compounds IV. Nucleophilic addition of alcohols and phenols to α-ethynyl ketones. J. Org. Chem. USSR 1972, 8, 1398–1402. [Google Scholar]
- Leonard, K.; Nelen, M.; Raghu, M.; Detty, M.R. Chalcogenopyranones from disodium chalcogenide additions to 1,4-pentadiyn-3-ones. The role of enol ethers as intermediates. J. Heterocycl. Chem. 1999, 36, 707–717. [Google Scholar] [CrossRef]
- Detty, M.R.; Murray, B.J.; Seidler, M.D. Preparation of 2,6-diphenyl-4H-chalcogenapyran-4-ones. J. Org. Chem. 1982, 47, 1968–1969. [Google Scholar] [CrossRef]
- Detty, M.R.; Hassnett, J.W.; Murray, B.J.; Reynolds, G.A. Δ4,4’-4-chalcogapyranyl-4H-chalcogenapyrans. Tetrahedron 1985, 41, 4853–4859. [Google Scholar] [CrossRef]
- Chantrapromma, S.; Ruanwas, P.; Boonnak, N.; Chantrapromma, K.; Fun, H.-K. Synthesis, antityrosinase activity of curcumin analogues, and crystal structure of (1E,4E)-1,5-bis(4-ethoxyphenyl)penta-1,4-dien-3-one. Crystallogr. Rep. 2016, 61, 1081–1085. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I. Monitoring initial structural changes in a crystal during photo-induced disappearance of its diffracting properties. Chem. Phys. 2003, 288, 241–247. [Google Scholar] [CrossRef]
- Cea-Olivares, R.; Rodriguez, I.; Rosales, M.J.; Toscano, R.A. The structure of triketones in the solid-state. The crystal structure of 1,5-diphenylpentane-1,3,5-trione. Aust. J. Chem. 1987, 40, 1127–1130. [Google Scholar] [CrossRef]
- Kusakawa, T.; Sakai, S.; Nakajima, K.; Yuge, H.; Rzeznicka, I.; Hori, A. Synthesis, structures and co-crystallizations of perfluorophenyl substituted β-diketone and triketone compounds. Crystals 2019, 9, 175. [Google Scholar] [CrossRef]
- Momeni, B.Z.; Fathi, N.; Rahimi, F.; Rominger, F. Structural features of two pyridyl compounds of 1,5-bis(2’-pyridyl)pentane-1,3,5-trione and a new salt of doubly protonated hydroxyterpyridinium. J. Chem. Crystallogr. 2020, 50, 77–87. [Google Scholar] [CrossRef]
- CrysAlisPro. Rigaku Oxford Diffraction, v1.171.43.109a; Rigaku Corporation: Tokyo, Japan, 2023.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| Bond Lengths | Angles | ||
|---|---|---|---|
| O(1)–C(1) | 1.368(3) | C(4)–C(1)–O(1) | 108.1(2) |
| C(1)–C(4) | 1.484(4) | O(1)–C(1)–C(2) | 122.6(2) |
| C(1)–C(2) | 1.346(4) | C(2)–C(1)–C(4) | 129.2(2) |
| C(2)–C(3) | 1.469(3) | C(1)–C(2)–C(3) | 129.1(3) |
| C(3)–O(3) | 1.230(4) | C(2)–C(3)–O(3) | 123.79(16) |
| C(2)–C(3)–C(2′) | 112.4(3) |
| D—H···A | D—H | H···A | D···A | D—H···A | Symmetry |
|---|---|---|---|---|---|
| C(8)–H(8)···O(3) * | 0.95 | 2.52 | 3.398(4) | 154 | 1−x, 2−y, 1−z |
| C(9)–H(9)···O(3) | 0.95 | 2.48 | 2.881(3) | 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cordes, D.B.; Kennett, V.; McKay, A.P. (E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one. Molbank 2025, 2025, M1986. https://doi.org/10.3390/M1986
Aitken RA, Cordes DB, Kennett V, McKay AP. (E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one. Molbank. 2025; 2025(2):M1986. https://doi.org/10.3390/M1986
Chicago/Turabian StyleAitken, R. Alan, David B. Cordes, Verity Kennett, and Aidan P. McKay. 2025. "(E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one" Molbank 2025, no. 2: M1986. https://doi.org/10.3390/M1986
APA StyleAitken, R. A., Cordes, D. B., Kennett, V., & McKay, A. P. (2025). (E,E)-1,5-Diethoxy-1,5-diphenylpenta-1,4-dien-3-one. Molbank, 2025(2), M1986. https://doi.org/10.3390/M1986










