Abstract
A highly novel method for the preparation of benzyl 2-phenyl-1H-pyrrole-1-carboxylate has been developed. The intramolecular reaction of benzyl (E)-(4-oxo-4-phenylbut-2-en-1-yl)carbamate with oxalyl chloride provided title compound in good yields. The structure of the newly synthesized compound was determined using 1H-, 13C-NMR, IR, and mass spectral data.
1. Introduction
Pyrrole is one of the most valuable structural scaffolds, frequently encountered in a variety of biologically active natural products [1,2], pharmaceuticals [3,4], and materials science and industry [5]. Well-established synthetic methods—such as the Paal–Knorr reaction [6], the Hantzsch reaction [7], and the Barton–Zard reaction [8]—have been widely employed to prepare biologically active pyrroles. Additionally, multicomponent reactions using transition-metal catalysis have been developed for synthesizing pyrrole derivatives [9,10]. Among these, functionalized 2-arylpyrroles serve as promising precursors for novel selective dopamine D3 receptor antagonists and BODIPY dyes or chromophores [11,12]. Accordingly, several strategies have been devised for 2-arylpyrrole synthesis, with Suzuki coupling being predominantly used for N-protected 2-arylpyrroles (Scheme 1) [13,14]. Recently, visible-light-mediated direct C–H arylation of N-protected pyrroles with aryl diazonium salts using a photoredox catalyst has also been employed for the synthesis of 2-arylpyrroles [15]. However, although N-Boc-protected 2-arylpyrroles have been extensively synthesized via Suzuki coupling, to our knowledge, N-Cbz-protected 2-arylpyrroles have not yet been reported. Herein, we describe the synthesis of benzyl 2-phenyl-1H-pyrrole-1-carboxylate through the intramolecular reaction of benzyl (E)-(4-oxo-4-phenylbut-2-en-1-yl)carbamate.
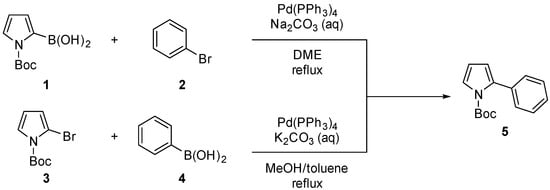
Scheme 1.
Synthesis of N-Boc-2-phenylprrole through Suzuki coupling.
2. Results and Discussion
In our previous studies [16], an asymmetric intramolecular Michael reaction of α,β-unsaturated carbonyl compounds containing benzyl alcohol afforded enantioenriched 1-substituted phthalans. Building on this work, we anticipated that a Lewis acid-catalyzed intramolecular reaction of an γ-amino-α,β-unsaturated carbonyl compound would yield pyrrole derivatives. Accordingly, we prepared benzyl (E)-(4-oxo-4-phenylbut-2-en-1-yl)carbamate (8) via the Wittig reaction of Cbz glycinal 6 with 1-phenyl-2-(triphenylphosphoranylidene)ethanone 7 with a 35% yield. Next, we carried out an intramolecular reaction of compound 8 using oxalyl chloride in MeOH at room temperature. In this reaction, HCl is generated in situ from SOCl₂ and MeOH, acting as a Lewis acid. This process successfully yielded the desired benzyl 2-phenyl-1H-pyrrole-1-carboxylate (9) with a 76% yield (Scheme 2). The structure of compound 9 was confirmed by by 1H-, and 13C-NMR, IR, and mass spectral data, all of which were consistent with the proposed structure.
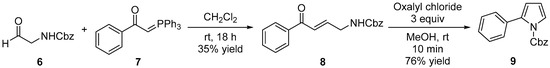
Scheme 2.
Synthesis of benzyl 2-phenyl-1H-pyrrole-1-carboxylate (9).
The structure of compounds 8 and 9 were confirmed by 1H-, 13C-NMR, IR, and mass spectral data, all of which were consistent with the proposed structure. All data are available in the Supplementary Materials (Figures S1–S6 and Tables S1 and S2).
3. Materials and Methods
3.1. General
All reagents were used as received without further purification. Chromatographic purification was accomplished using forced-flow chromatography on ICN 60 32–64 mesh silica gel 63 (Merck, Darmstadt, Germany). Thin-layer chromatography (TLC) (Merck, Darmstadt, Germany) was performed on EM Reagents 0.25 mm silica gel 60-F plates. Developed chromatograms were visualized by fluorescence quenching and anisaldehyde staining. 1H- and 13C-NMR spectra were recorded on 400 MHz instrument (Bruker BioSpin GmbH, Karlsruhe, Germany) as noted, and were internally referenced to residual protio solvent signals. Data for 1H NMR were reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, dd = doublet of doublets), integration, coupling constant (Hz), and assignment. Data for 13C-NMR were reported in terms of chemical shift. IR spectra were recorded on Perkin–Elmer 1600 FT-IR spectrometer (Bruker Optics GmbH, Ettlingen, Germany), and reported in terms of frequency of absorption (cm−1). High-resolution mass spectrometry data were recorded on a JEOL JMS-700 M Station mass spectrometer (JEOL, Tokyo, Japan).
3.2. Syntheis of (E)-(4-Oxo-4-phenylbut-2-en-1-yl)carbamate (8)
A solution of benzyl (2-oxoethyl)carbamate (6, 0.97 g, 5 mmol, 1 equiv) in CH2Cl2 (25 mL, 0.2 M) was added to 1-phenyl-2-(triphenylphosphoranylidene)ethanone (7, 1.9 g, 5 mmol, 1.0 equiv). The reaction mixture was stirred at room temperature for 18 h. Then, the resulting mixture was concentrated in vacuo and was purified by flash column chromatography with EtOAc/hexanes (1/4) as an eluent to obtain desired product 8 (35%, 0.52 g). White solid; m.p. 63–65 °C; 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 7.4 Hz, 2H), 7.60–7.53 (m, 1H), 7.48 (d, J = 1.5 Hz, 2H), 7.41–7.36 (m, 3H), 7.36 (s, 2H), 6.98 (s, 2H), 5.15 (s, 2H), 5.05 (s, 1H), 4.14–4.03 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 190.3, 156.2, 144.2, 137.5, 136.3, 133.0, 128.64 (two peaks overlapping), 128.60, 128.3, 128.2, 125.6, 67.1, 42.3; IR (neat) 3553, 3056, 3035, 2930, 2898, 1714, 1692, 1670, 1625, 1537, 1486, 1254, 1241, 1211, 1177, 1151, 1053, 1021 cm−1; HRMS (EI) m/z calcd for [M]+ C18H17NO3: 295.1208 Found: 295.1180.
3.3. Syntheis of Benzyl 2-Phenyl-1H-pyrrole-1-carboxylate (9)
A solution of benzyl (E)-(4-oxo-4-phenylbut-2-en-1-yl)carbamate (8, 30 mg, 0.1 mmol, 1 equiv) in MeOH (1.0 mL, 0.1 M) was added to oxalyl chloride (26 μL, 0.3 mmol, 3.0 equiv). After stirring at room temperature for 10 min, the reaction mixture was quenched with water and was extracted with CH2Cl2. Afterwards, the combined organic layers was washed with brine and dried over Na2CO3. Then, the resulting mixture was concentrated in vacuo and was purified by flash column chromatography with EtOAc/hexanes (1/20) as an eluent to obtain desired product 9 (76%, 21 mg). Colorless oil; 1H NMR (400 MHz, CDCl3) δ 7.39 (dd, J = 3.4, 1.8 Hz, 1H), 7.37–7.33 (m, 2H), 7.33–7.28 (m, 6H), 7.20 (dd, J = 6.6, 2.9 Hz, 2H), 6.25 (t, J = 3.3 Hz, 1H), 6.22 (dd, J = 3.3, 1.8 Hz, 1H), 5.22 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 150.7, 135.7, 134.8, 133.8, 129.4, 128.7, 128.6, 128.4, 127.7, 127.5, 122.6, 115.1, 111.5, 68.9; IR (neat) 1784, 1732, 1714, 1399, 1381, 1328, 1293, 1215, 1179, 1147, 1112, 1074, 1042, 1026, 1001, cm−1; HRMS (EI) m/z calcd for [M]+ C18H15NO2: 277.1103 Found: 277.1121.
Supplementary Materials
Figure S1: 1H NMR spectrum of compound 8, Figure S2: 13C NMR spectrum of compound 8, Figure S3: 1H NMR spectrum of compound 9, Figure S4: 13C NMR spectrum of compound 9, Figure S5: IR spectra of compound 8, Table S1: High mass data of compound 8, Figure S6: IR spectra of compound 9, Table S2: High mass data of compound 9.
Funding
This work was supported by Kyonggi University Research Grant 2024.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Young, I.S.; Thornton, P.D.; Thompson, A. Synthesis of Natural Products Containing the Pyrrolic Ring. Nat. Prod. Rep. 2010, 27, 1801–1839. [Google Scholar] [PubMed]
- Singh, N.; Singh, S.; Kohli, S.; Singh, A.; Asiki, H.; Rathee, G.; Chandra, R.; Anderson, E.A. Recent Progress in the Total Synthesis of Pyrrole-Containing Natural Products (2011–2020). Org. Chem. Front. 2021, 8, 5550–5573. [Google Scholar]
- Ahmad, S.; Alam, O.; Naim, M.J.; Shaquiquzzaman, M.; Alam, M.M.; Iqbal, M. Pyrrole: An Insight into Recent Pharmacological Advances with Structure Activity Relationship. Eur. J. Med. Chem. 2018, 157, 527–561. [Google Scholar] [PubMed]
- Ganesh, B.H.; Raj, A.G.; Aruchamy, B.; Nanjan, P.; Drago, C.; Ramani, P. Pyrrole: A Decisive Scaffold for the Development of Therapeutic Agents and Structure-Activity Relationship. ChemMedChem 2024, 19, e202300447. [Google Scholar] [CrossRef] [PubMed]
- Dydio, P.; Lichosyt, D.; Jurczak, J. Amide- and Urea-Functionalized Pyrroles and Benzopyrroles as Synthetic, Neutral Anion Receptors. Chem. Soc. Rev. 2011, 40, 2971–2985. [Google Scholar] [PubMed]
- Khaghaninejad, S.; Heravi, M.M. Paal–Knorr Reaction in the Synthesis of Heterocyclic Compounds. Adv. Heterocycl. Chem. 2014, 111, 95–146. [Google Scholar]
- Leonardi, M.; Estévez, V.; Villacampa, M.; Menéndez, J. The Hantzsch Pyrrole Synthesis: Non-Conventional Variations and Applications of a Neglected Classical Reaction. Synthesis 2019, 51, 816–828. [Google Scholar]
- Barton, D.H.R.; Kervagoret, J.; Zard, S.Z. A Useful Synthesis of Pyrroles from Nitroolefins. Tetrahedron 1990, 46, 7587–7598. [Google Scholar] [CrossRef]
- Estévez, V.; Villacampa, M.; Menéndez, J.C. Multicomponent reactions for the synthesis of pyrroles. Chem. Rev. 2004, 104, 2127–2198. [Google Scholar]
- Abu Sohel, S.M.; Liu, R.-S. Carbocyclisation of Alkynes with External Nucleophiles Catalysed by Gold, Platinum and Other Electrophilic Metals. Chem. Soc. Rev. 2010, 39, 4402–4421. [Google Scholar]
- Boyfield, I.; Coldwell, M.C.; Hadley, M.S.; Healy, M.A.M.; Johnson, C.N.; Nash, D.J.; Riley, G.J.; Scott, E.E.; Smith, S.A.; Stemp, G. Novel 2,5-disubstituted-1H-pyrroles with high affinity for the dopamine D3 receptor: N-benzyl modifications. Bioorg. Med. Chem. Lett. 1997, 7, 327–330. [Google Scholar]
- De Bonfils, P.; Péault, L.; Nun, P.; Coeffard, V. State of the Art of Bodipy-Based Photocatalysts in Organic Synthesis. Eur. J. Org. Chem. 2021, 12, 1809–1824. [Google Scholar]
- Johnson, C.N.; Stemp, G.; Anand, N.; Stephen, S.C.; Gallagher, T. Palladium (0)-Catalysed Arylations using Pyrrole and Indole 2-Boronic Acids. Synlett 1998, 1025–1027. [Google Scholar]
- Yilmaz, R.F.; Derin, Y.; Misir, B.A.; Atalay, V.E.; Tutar, Ö.F.; Ökten, S.; Tutar, A. Synthesis and spectral properties of symmetrically arylated BODIPY dyes: Experimental and computational approach. J. Mol. Struct. 2023, 1291, 135962. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Z.; Bao, W.; Li, J.; Guo, B.; Huang, S.; Zhang, Y.; Rao, Y. Perylenequinonoid-catalyzed photoredox activation for the direct arylation of (het)arenes with sunlight. Org. Biomol. Chem. 2019, 17, 4364–4369. [Google Scholar] [PubMed]
- Son, E.C.; Kim, S.Y.; Kim, S.-G. Squaramide-Catalyzed Asymmetric Intramolecular Oxa-Michael Reaction of α,β-Unsaturated Carbonyls Containing Benzyl Alcohol: Construction of Chiral 1-Substituted Phthalans. J. Org. Chem. 2021, 86, 6826–6839. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).